Abstract
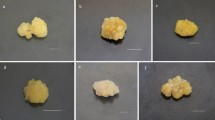
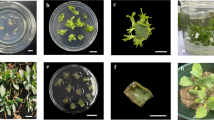
The present study aims to analyze the effect of bacterial volatiles on tobacco callus organogenesis. A glass prototype was designed in the shape of the letter ‘H’ such that plants and bacteria could be grown in physically separated compartments while volatile compounds could still be exchanged. Volatiles from a beneficial bacterium, Bacillus badius M12, which showed growth promotion in Sesamum indicum (sesame), also induced organogenesis in tobacco callus. Volatiles from B. badius M12 appeared to maintain tobacco callus in a green and viable state during the prolonged, 45 d, sealed incubation. This apparent antioxidant effect was investigated by exposing freshly cut cylinders of apple flesh, a tissue noted for its ready tendency to oxidize, to bacterial volatiles. The expression of superoxide dismutase (SOD), a key antioxidant enzyme, was higher in callus exposed to bacterial volatiles, confirming that they enhanced antioxidant activity. Exposure of apple tissues to volatile compounds from B. badius M12 and a well-known growth-promoting bacterial volatile, 2,3-butanediol, led to increased antioxidant activity. We conclude that plant growth-promoting bacterial volatiles were useful in tissue organogenesis and increased antioxidant activity.





Similar content being viewed by others
References
Alstrom S, Burns RG (1989) Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol Fertil Soils 7:232–238
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457
Bashan Y, Holguin G (2002) Plant growth-promoting bacteria: a potential tool for arid mangrove reforestation. Trees 16:159–166
Beauchump C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:275–287
Bedigian D, Harlan J (1986) Evidence for cultivation of sesame in the ancient world. Econ Bot 40:137–154
Benson EE (2000) Do free radicals have a role in plant tissue culture recalcitrance? In Vitro Cell Dev Plant 36:163–170
Blom D, Fabbri C, Eberl L, Weisskopf L (2011) Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 77:1000–1008. doi:10.1128/AEM.01968-10
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bray HG, Thorpe WV (1954) Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal 1:27–52
Chanjirakul K, Wang SY, Wang CY, Siriphanich J (2006) Effect of natural volatile compounds on antioxidant capacity and antioxidant enzymes in raspberries. Postharvest Biol Technol 40:106–115. doi:10.1016/j.postharvbio.2006.01.004
Chanjirakul K, Wang SY, Wang CY, Siriphanich J (2007) Natural volatile treatments increase free-radical scavenging capacity of strawberries and blackberries. J Sci Food Agric 87:1463–1472. doi:10.1002/jsfa
Cheung SCM, Szeto YT, Benzie IFF (2007) Antioxidant protection of edible oils. Plant Food Hum Nutr 62:39–42
Cho SM, Kang BR, Han SH, Anderson AJ, Park J-Y, Lee Y-H, Cho BH, Yang K-Y, Ryu C-M, Kim YC (2008) 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol Plant Microbe Interact 21:1067–1075. doi:10.1094/MPMI-21-8-1067
Dan Y (2008) Biological functions of antioxidants in plant transformation. In Vitro Cell Dev Plant 44:149–161. doi:10.1007/s11627-008-9110-9
Digiacomo F, Girelli G, Aor B, Marchioretti C, Pedrotti M, Perli T, Tonon E, Valentini V, Avi D, Ferrentino G, Dorigato A, Torre P, Jousson O, Mansy SS, Bianco CD (2014) Ethylene-producing bacteria that ripen fruit. ACS Synth Biol 3:935–938
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198:16–32
Dunkel M, Schmidt U, Struck S, Berger L, Gruening B, Hossbach J, Jaeger IS, Effmert U, Piechulla B, Eriksson R, Knudsen J, Preissner R (2009) Super scent—a database of flavors and scents. Nucleic Acids Res 37:D291–D294
Farag MA, Ryu C-M, Sumner LW, Paré PW (2006) GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochem 67:2262–2268
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17:507–523. doi:10.1111/j.1365-3040.1994.tb00146.x
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
He Y, Guo X, Lu R, Niu B, Pasapula V, Hou P, Cai F, Xu Y, Chen F (2009) Changes in morphology and biochemical indices in browning callus derived from Jatropha curcas hypocotyls. Plant Cell Tissue Org 98:11–7. doi:10.1007/s11240-009-9533-y
Holguin G, Vazquez P, Bashan Y (2001) The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils 33:265–278
Ibarz A, Paga J, Garza S (2000) Kinetic models of non-enzymatic browning in apple puree. J Sci Food Agric 80:1162–1168
Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B (2009) Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81:1001–012
Karadeniz A, Topcuoğlu ŞF, İnan S (2006) Auxin, gibberellin, cytokinin and abscisic acid production in some bacteria. World J Microbiol Biotechnol 22:1061–1064
Khan MS, Zaidi A, Wani PA, Ahemad M, Oves M (2009) Functional diversity among plant growth-promoting rhizobacteria: current status. Springer, Netherland
Kim YC, Leveau J, Mcspadden Gardener BB, Pierson EA, Pierson LS III, C-m R (2011) The multifactorial basis for plant health promotion by plant-associated bacteria. Appl Environ Microbiol 77:1548–1555. doi:10.1128/AEM.01867-10
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Kloepper JW, Rodriguez-Kabana R, Zehnder GW, Murphy J, Sikora E, Fernandez C (1999) Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Aust J Plant Pathol 28:27–33
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathol 94:1259–1266
Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B (2014) mVOC: a database of microbial volatiles. Nucleic Acids Res 42:D744–D748
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact 20:207–217
McIlvain TC (1921) A buffer solution for colorimetric comparison. J Biol Chem 49:183–86
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Ozoglu H, Bayndrl A (2002) Inhibition of enzymic browning in cloudy apple juice with selected antibrowning agents. Food Control 13:213–221
Palanisami S, Prabaharan D, Uma L (2009) Fate of few pesticide-metabolizing enzymes in the marine cyanobacterium Phormidium valderianum BDU 20041 in perspective with chlorpyrifos exposure. Pestic Biochem Physiol 94:68–72
Pieterse CMJ, van Wees SCM, Ton J, van Pelt JA, van Loon LC (2002) Signalling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biol 4:535–544
Ravikumar S, Kathiresan K, Ignatiammal TM, Selvam MB, Shanty S (2004) Nitrogen-fixing azotobacters from mangrove habitat and their utility as marine biofertilizers. J Exp Mar Biol Ecol 312:5–7
Rudrappa T, Splaine RE, Biedrzycki ML, Bais HP (2008) Cyanogenic pseudomonads influence multitrophic interactions in the rhizosphere. PLoS One 3:e2073. doi:10.1371/journal.pone.0002073
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932
Ryu C-M, Farag MA, Paré PW, Kloepper JW (2005) Invisible signals from the underground: bacterial volatiles elicit plant growth promotion and induced systemic resistance. Plant Pathol J 21:7–12
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Prod Rep 24:814–842
Song GC, Ryu C-M (2013) Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int J Mol Sci 14:9803–9819
Sundararaman M, Boopathi T, Gopinath S (2007) Status of mangrove ecosystem: exploring the potential role of cyanobacteria in restoration and afforestation. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments. Springer, Netherland, pp 209–224
Tronc J-S, Lamarche F, Makhlouf J (1997) Enzymatic browning inhibition in cloudy apple juice by electrodialysis. J Food Sci 62:75–79
Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Fertil Soils 30:460–468
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73:5639–5641
Wani PA, Khan MS, Zaidi A (2007) Chromium reduction, plant growth-promoting potentials, and metal solubilizatrion by Bacillus sp. isolated from alluvial soil. Curr Microbiol 54:237–243
Wei W, Qi X, Wang L, Zhang Y, Hua W, Li D, Lv H, Zhang X (2011) Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genomics 12:451
Xie X, Zhang H, Paré PW (2009) Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03). Plant Signal Behav 4:948–953
Zacchini M, de Agazio M (2004) Spread of oxidative damage and antioxidative response through cell layers of tobacco callus after UV-C treatment. Plant Physiol Biochem 42:445–450. doi:10.1016/j.plaphy.2004.03.007
Zhang H, Kim M-S, Sun Y, Dowd SE, Shi H, Paré PW (2008) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant Microbe Interact 21:737–744. doi:10.1094/MPMI-21-6-0737
Acknowledgments
This research work was financially supported by the Ministry of Environment and Forests (MoEF), Govt. of India. We would like to thank Dr. T. Boopathi for his help during experiments and manuscript preparation. We are thankful to Dr. N. Jayabalan, Department of Plant Sciences, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India and his research students for providing plant seeds and for helping during callus preparation. Language editing by Dr. Carla A. Spence, Department of Plant and Soil Sciences, University of Delaware, Newark, Delaware, USA has greatly improvised our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 10927 kb)
Rights and permissions
About this article
Cite this article
Gopinath, S., Kumaran, K.S. & Sundararaman, M. A new initiative in micropropagation: airborne bacterial volatiles modulate organogenesis and antioxidant activity in tobacco (Nicotiana tabacum L.) callus. In Vitro Cell.Dev.Biol.-Plant 51, 514–523 (2015). https://doi.org/10.1007/s11627-015-9717-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9717-6