Abstract
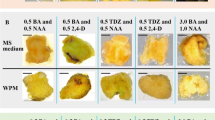
We describe an efficient protocol for callus induction from adult tissues of Prunus persica (L.) Batsch. Three different commercial peach genotypes, Early May®, Zise May®, and UFO-3®, plus three other genotypes from hybrid crosses performed in February 2006, PS108, PS208, and PS708, were used in the study. Thirteen explant treatments were tested using nine different plant parts. Murashige and Skoog and woody plant medium salts were assayed with several concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D), kinetin (KN), and thidiazuron, and two different photoperiods were tested, a 16-h photoperiod or continuous darkness. In terms of the quantitative response, two parameters were assessed: the number of d to callus induction and relative callus growth recorded after 30 d. Woody plant medium supplemented with 2,4-D and KN significantly increased the rates of callus induction in the majority of treatments. And no significant differences among the P. persica genotypes were found. The explants derived from stem and calyx produced up to 85 and 96% callus induction, respectively. The protocol described here could be used for efficient callus induction in a range of Prunus spp.


Similar content being viewed by others
References
Abbott AG, Arús P, Scorza R (2008) Genetic engineering and genomics. In: Layne DR, Bassi D (eds) The peach: botany, production and uses. CABI, Wallingford, pp 85–105
Ansley PJ, Collins GG, Sedgley M (2000) Adventitious shoot regeneration from leaf explants of almond (Prunus dulcis Mill.). In Vitro Cell Dev Biol Plant 36:470–474
Arora R, Wisniewski ME (1995) Ultrastructural and protein changes in cell suspension cultures of peach associated with low temperature-induced cold acclimation and abscisic acid treatment. Plant Cell Tiss Organ Cult 40:17–24
Bhansali RR, Driver JA, Durzan DJ (1991) Somatic embryogenesis in cell suspension cultures of Prunus persica (L.). J Hortic Sci 66:601–605
Declerck V, Korban SS (1996) Influence of growth regulators and carbon sources on callus induction, growth and morphogenesis from leaf tissues of peach (Prunus persica L. Batsch). J Hortic Sci 71:49–55
Gentile A, Monticelli S, Damiano C (2002) Adventitious shoot regeneration in peach (Prunus persica (L.) Batsch). Plant Cell Rep 20:1011–1016
George EF (1993) Plant propagation by tissue culture. Part 1. The technology. Exegetics Ltd, Edington, p 14
Green CE, Phillips RL (1975) Plant regeneration from tissue cultures of maize. Crop Sci 15:417–427
Guiderdoni E, Demarly Y (1988) Histology of somatic embryogenesis in cultured leaf segments of sugarcane plantlets. Plant Cell Tiss Organ Cult 14:71–88
Hammerschlag FA, Bauchan G, Scorza R (1985) Regeneration of peach plants from callus derived from immature embryos. Theor Appl Genet 70:248–251
Hidano Y, Niizeki M (1988) Protoplast culture of deciduous fruit tree. Sci Hortic 37:201–216
Işikalan Ç, Akbas F, Namli S, Başaram D (2010) Adventitious shoot development from leaf and stem explants of Amygdalus communis L. cv. Yaltinski. Plant Omics J 3:92–96
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proc Int Plant Propag Soc 30:421–427
Long CM, Mulinix CA, Iezzoni AF (1994) Production of a microspore-derived callus population from sweet cherry. HortSci 29:1346–1348
López-Pérez AJ, Carreño J, Martínez-Cutillas A, Dabauza M (2005) High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. Vitis 44:79–85
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Paris R, Pratesi D, Negri P (2004) In vitro morphogenic ability of mature or embryonic apricot tissues. Acta Horticult 663:487–490
Peixe A, Barroso J, Potes A, Pais MS (2004) Induction of haploid morphogenic calluses from in vitro cultured anthers of Prunus armeniaca cv. ‘Harcot’. Plant Cell Tiss Organ Cult 77:35–41
Pérez-Jiménez M, Carrillo-Navarro A, Cos-Terrer J (2012) Regeneration of peach (Prunus persica L. Batsch) cultivars and Prunus persica × Prunus dulcis rootstocks via organogenesis. Plant Cell Tiss Organ Cult 108:55–62
Scorza R, Ravelonandro M, Callahan AM, Cordts JM, Fuchs M, Dunez J, Gonsalves D (1994) Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Rep 14:18–22
Svircev AM, Biggs AR, Miles NW (1993) Peach regeneration from callus derived from embryos of selected cultivars. Fruit Var J 47:13–16
Zhou H, Li M, Zhao X, Fan X, Guo A (2010) Plant regeneration from in vitro leaves of the peach rootstock ‘Nemaguard’ (Prunus persica × P. davidiana). Plant Cell Tiss Organ Cult 101:79–87
Acknowledgments
This research was supported by the Instituto Nacional de Investigaciones Agrarias (INIA) (RTA2008-00121-00-00) and by a fellowship provided by INIA to Margarita Pérez-Jiménez.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Pérez-Jiménez, M., López-Soto, M.B. & Cos-Terrer, J. In vitro callus induction from adult tissues of peach (Prunus persica L. Batsch). In Vitro Cell.Dev.Biol.-Plant 49, 79–84 (2013). https://doi.org/10.1007/s11627-012-9466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9466-8