Summary
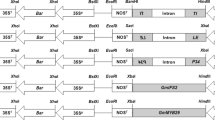
We have developed a new transformation method called MATVS (Multi-Auto-Transformation Vector System). The oncogenes (ipt or rol genes) of Agrobacterium are used as selectable markers to regenerate transgenic cells and to select marker-free transgenic plants in the MATVS. The chimeric ipt gene or the rol genes are combined withthe site-specific recombination R/RS system to remove the oncogenes from the transgenic cells after transformation. We report here the application of MATVS to transformation of tobacco, aspen, rice and snapdragon. (I) The GST-MAT vector pMAT8 has the native ipt gene and the R gene with a chemical inducible promoter (GST-II-27). Use of the GST-MAT vector generated marker-free transgenic tobacco plants cotaining a single copy transgene at high frequency. (2) Use of the GST-MAT vector pRBI11 containing the rbcS 3B-ipt gene produced transgenic marker-free hybrid aspen plants without crossing. (3) Use of the ipt-type MAT vector, pNPI30GFP, containing the 35S-ipt and 35S-R, genes, resulted in the regeneration of marker-free transgenic reice plants directly from infected scutellum tisues at high frequency within 1 mo. (4) Use of the rol-type MAT vector pNPI702, containing the rol genes and the 35S-R gene, produced transgenic marker-free plants of tobacco and snapdragon at high frequency without crossing. Our results show that the promoter of the ipt gene, the preculture periods of plant tissues and the culture medium are important factors to improve the generation efficiency of marker-free transgenic plants. We can rapidly produce marker-free transgenic plants without the production of ipt-shooty intermediates. Therefore, it is a most promising method to save time and work for the generation of marker-free transgenic plants in crops.
Similar content being viewed by others
References
Akiyoshi, D. E.; Klee, H.; Amasino, R. M.; Nester, E. W.; Gordon, M. P. T DNA of Agrobacterium tumerfaciens encode an enzyme of eytokinin biosynthesis. Proc. Natl Acad. Sci. USA 81:5994–5998; 1984.
Araki, H.; Jearnpipatkul, A.; Tatsumi, H.; Sakurai, T.; Ushino, K.; Muta, T.; Oshima, Y. Molecular and functional organization of yeast plasmid pSRI. J. Mol. Biol. 182:191–203; 1987.
Barry, G. F.; Rogers, S. G.; Fraley, R. T.; Brand, L. Identification of a cloned cytokinin biosynthetic gene. Proc. Natl Acad. Sci. USA 81:4776–4780; 1984.
Boulter, M. E.; Croy, E.; Simpson, P.; Shields, R.; Croy, R. R. D.; Shirsat, A. H. Transformation of Brassica napus L. (oilseed rape) using Agrobacterium tumefaciens and Agrobacterium rhizogenes—a comparison. Plant Sci. 70:91–99; 1990.
Christey, M. C. Transgenic crop plants using Agrobacterium rhizogenes-mediated transfromation. In: Doran, P. M., ed. Hairy roots: culture and application. Amsterdam: Hardwood Academic Publishers; 1997:99–111.
Christou, P. Transformation technology. Trends Plant Sci. 1:423–431; 1996.
Cui, M.; Takayanagi, K.; Kamada, H.; Nishimura, S.; Handa, T. Transformation of Antirrhinum majus L. by a rol-type multi-autotransformation (MAT) vector system. Plant Sci. 159:273–280; 2000.
Cui, M.; Takayanagi, K.; Kamada, H.; Nishimura, S.; Handa, T. Efficient shoot regenration from hairy roots of Antirrhinum majus L. transformed by rol type MAT vector system. Plant. Cell Rep. In press.
Dale, E. C.; Ow, D. W. Gene transfer with subsequent removal of the selection gene from the host genome. Proc. Natl Acad. Sci. USA. 88:10558–10562; 1991.
Ebinuma, H.; Mastsunaga, E.; Yamada, K.; Yamakado, M. Transformation of hybrid aspen for resistance to crown gall disease. In: Klopfenstein, N. B.; Chun, Y. W.; Kim, M.-S.; Ahuja, M. R., eds. Micropropagation, genetic engineering, and molecular biology of populus. Gen. Tech. Rep. RM-GTR-297. Fort Collins, USA: Rocky Mountain Forest and Range Experimental Station; 1997a:161–164.
Ebinuma, H.; Sugita, K.; Matsunaga, E.; Yamakado, M. Selection of markerfree transgenic plants using the isopentenyl transferase gene as a selectable marker. Proc. Natl. Acad. Sci. USA 94:2117–2121; 1997b.
Ebinuma, H.; Sugita, K.; Matsunaga, E.; Endo, S.; Kasahara, T. Selection of marker-free transgenic plants using the oncogenes (ipt, rol, A, B, C) of Agrobacterium as selectable markers. In: Jain, S. M.; Minocha, S. C.; eds. Molecular biology of woody plants II. Dordreclit, The Netherlands: Kluwer Academic Publishers; 2000:25–46.
Ebinuma, H.; Sugita, K.; Matsumaga, E.; Yamakado, M.; Komamine, A. Principle of MAT vector. Plant Biotech. 14:133–139; 1997c.
Endo, S.; Kasahara, T.; Sugita, K.; Matsunaga, E.; Ebinuma, H. The isopentenyl transferase gene is effective as a selectable marker gene for plant transformation. Plant Cell Rep. In press; 1999.
Gaudin, V.; Vrain, T.; Jouanin, L. Bacterial genes modifying hormonal balances in plants. Plant Physiol. Biochem. 32:11–29; 1994.
Gleave, A. P.; Mitra, D. S.; Morris, B. A. M. Selectable marker-free transgenic plants without sexual crossing: transient expression of Cre recombinase and use of a conditional lethal dominant gene. Plant Mol. Biol. 40:223–235; 1999.
Haldrup, A.; Petersen, S. G.; Okkels, F. T. Positive selection: a plant selection principle based on xylose isomerase, an enzyme used in the food industry. Plant Cell Rep. 18:76–81; 1998.
Hansen, G.; Wright, M. S. Recent advances in the transformation of plants. Trends Plant Sci. 4:226–231; 1999.
Hatamoto, H.; Boulter, M. E.; Shirsat, A. H.; Croy, E. J.; Ellis, J. R. Recovery of morphologically normal transgenic tobacco from hairy roots co-transformed with Agrobacterium rhizogenes and a binary vector plasmid. Plant Cell Rep. 9:88–92; 1990.
Hiei, Y.; Komari, T.; Kubo, T. Transformation of rice mediated by Agrobacterium tumerfaciens. Plant Mol. Biol. 35:205–218; 1997.
Holt, D. C.; Lay, V. J.; Clarke, E. D.; Dinsmore, A.; Jepson, I.; Bright, S. W. J.; Greenland, A. J. Characterization of the safener-induced glutathione S-transferase isoform II from maize. Planta 196:295–302; 1995.
Hooykaas, P. J. J.; Schilperoort, R. A. Agrobacterium and plant genetic engineering. Plant Mol. Biol. 19:15–38; 1992.
Joersbo, M.; Donaldson, I.; Kreiberg, J.; Petersen, S. G.; Brunstedt, J.; Okkels, F. T. Analysis of mannose selection used for transformation of sugar beet. Mol. Breed. 4:111–117; 1998.
Kiyokawa, S.; Kobayashi, K.; Kikuchi, Y.; Kamada, H.; Harada, H. Root-inducing region of mikimopine type Ri plasmid pRi724. Plant Physiol. 104:801–802; 1994.
Komari, T.; Hiei, Y.; Saito, Y.; Murai, N.; Kumashiro, T. Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 10:165–174; 1996.
Kunkel, T.; Niu, Q.-W.; Chan, Y.-S.; Chua, N.-H. Inducible isopentenyl transferase as a high-efficiency marker for plant transformation. Nat. Biotechnol. 17:916–919; 1999.
Morten, J.; Okkels, F. A novel principle for selection of transgenic plant cells: positive selection. Plant Cell Rep. 16:219–221; 1999.
Negrotto, D.; Jolley, M.; Beer, S.; Wenck, A. R.; Hansen, G. The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep. 19:798–803; 2000.
Odell, J.; Caimi, P.; Sauer, B.; Russell, S. Site-directed recombination in the genome of transgenic tobacco. Mol. Gen. Genet. 223:369–378; 1990.
Onouchi, H.; Nishihama, R.; Kudo, M.; Machida, Y.; Machida, C. Visualization of site-specific recombination catalyzed by a recombinase from Zygosaccharomyces rouxii in Arabidopsis thaliana. Mol. Gen. Genet. 247:653–660; 1995.
Onouchi, H.; Yokoi, K.; Machida, C.; Matsuzaki, H.; Oshima, Y.; Matsuoka, K.; Nakamura, K.; Machida, Y. Operation of an efficient site-specific recombination system of Zygosaccharomyces rouxii in tobacco cells. Nucl. Acids Res. 19:6373–6378; 1991.
Russell, S. H.; Hoopes, J. L.; Odell, J. L. Directed excision of a transgene from the plant genome. Mol. Gen. Genet. 234:49–59; 1992.
Schocher, R. J.; Shillito, R. D.; Saul, M. W.; Paszkowski, J.; Potrykus, I. Cotransformation of unlinked foreign genes into plants by direct gene transfer. Bio/Technology 4:1093–1096; 1986.
Slightom, J. L.; Durand-Tardif, M.; Jouanin, L.; Tepfer, D. Nucleotide sequence analysis of the TL-DNA of Agrobacterium rhizogenes type plasmid. J. Biol. 261:108–121; 1986.
Smigocki, A. C.; Owens, L. D. Cytokinin gene fused with a strong promoter enhances shoot organogenesis and zeatin levels in transformed plant cells. Proc. Natl Acad. Sci. USA. 85:5131–5135; 1988.
Sugita, M.; Gruissem, W. Developmenta, organ-specific, and lightdependent expression of the tomato ribulose-1,5-bisphosphate carboxylase small subunit gene family. Proc. Natl Acad. Sci. USA. 84:7104–7108; 1987.
Sugita, K.; Kasahara, T.; Matsunaga, E.; Ebinuma, H. A transformation vector for the production of marker-free transgenic plants containing a single copy transgene at high frequency. Plant J. 22:461–469; 2000a.
Sugita, K.; Matsunaga, E.; Ebinuma, H. Effective selection system for generating marker-free transgenic plants independent of sexual crossing. Plant Cell Rep. 18:941–947; 1999.
Sugita, K.; Matsunaga, E.; Kasahara, T.; Ebinuma, H. Transgene stacking in plants in the absence of sexual crossing. Mol. Breed. 6:529–536; 2000b.
Takayama, Y. Studies on the breeding of aspens (I): height growth in the early stage of F1 seedlings of Populus sieboldii Miq.×P. grandidentata Michx., Nichirinshi 50:267–273; 1968.
Tepfer, D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 37:959–967; 1984.
Van der Salm, T. P. M.; Hänisch ten Cate, Ch. H.; Dons, H. J. M. Prospects for applications of rol genes for crop improvement, Plant Mol. Biol. Rep. 14:207–228; 1996.
Wabiko, H.; Kagaya, M.; Kodama, I.; Masuda, K.; Kodama, Y.; Yamamoto, H.; Shibano, Y.; Sano, H. Isolation and characterization of diverse nopaline type Ti plasmids of Agrobacterium tumefaciens from Japan. Arch. Microbiol. 152:119–124; 1989.
White, F. F.; Taylor, B. H.; Huffman, G. A.; Gordon, M. P. Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J. Bacteriol. 164:33–44; 1985.
Yoder, J. I.; Goldsbrough, A. P. Transformation systems for generating marker-free transgenic plants. Bio/Technology 12:263–267; 1994.
Zubko, E.; Scutt, C.; Meyer, P. Intrachromosomal recombination between attP regions as a tool to remove selectable marker genes from tobacco transgenes. Nat. Biotechnol. 18:442–445; 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebinuma, H., Komamine, A. Mat (Multi-Auto-Transformation) vector system. The oncogenes of Agrobacterium as positive markers for regeneration and selection of marker-free transgenic plants. In Vitro Cell.Dev.Biol.-Plant 37, 103–113 (2001). https://doi.org/10.1007/s11627-001-0021-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11627-001-0021-2