Abstract
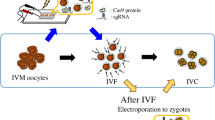
The present study was designed to investigate the effects of voltage strength on embryonic developmental rate and mutation efficiency in bovine putative zygotes during electroporation with the CRISPR/Cas9 system to target the MSTN gene at different time points after insemination. Results showed that there was no significant interaction between electroporation time and voltage strength on the embryonic cleavage and blastocyst formation rates. However, increasing the voltage strength to 20 V/mm to electroporate the zygotes at 10 h after the start of insemination yielded significantly lower blastocyst formation rates (P < 0.05) than those of the 10-V/mm electroporated zygotes. Mutation efficiency was then assessed in individual blastocysts by DNA sequence analysis of the target sites in the MSTN gene. A positive correlation between mutation rate and voltage strength was observed. The mutation efficiency in mutant blastocysts was significantly higher in the zygotes electroporated with 20 V/mm at 10 h after the start of insemination (P < 0.05) than in the zygotes electroporated at 15 h, irrespective of the voltage strength. We also noted that a certain number of blastocysts from zygotes that were electroporated with more than 15 V/mm at 10 h (4.8–16.7%) and 20 V/mm at 15 h (4.8%) were biallelic mutants. Our results suggest that the voltage strength during electroporation as well as electroporation time certainly have effects on the embryonic developmental rate and mutation efficiency in bovine putative zygotes.


Similar content being viewed by others
References
Abud HE, Lock P, Heath JK (2004) Efficient gene transfer into the epithelial cell layer of embryonic mouse intestine using low-voltage electroporation. Gastroenterology 126:1779–1787
Anderson EM, Haupt A, Schiel JA, Chou E, Machado HB, Strezoska Z, Lenger S, McClelland S, Birmingham A, Vermeulen A, Smith A (2015) Systematic analysis of CRISPR-Cas9 mismatch tolerance reveals low levels of off-target activity. J Biotechnol 211:56–65
Booth PJ, Tan S, Reipurth R, Holm P, Callesen H (2001) Simplification of bovine somatic cell nuclear transfer by application of a zona-free manipulation technique. Cloning & Stem Cells 3:139–150
Bosch P, Hodges CA, Stice SL (2004) Generation of transgenic livestock by somatic cell nuclear transfer. Biotecnol Apl 21:128–136
Boverhof DR, Chamberlain MP, Elcombe CR, Gonzalez FJ, Heflich RH, Hernandez LG, Jacobs AC, Jacobson-Kram D, Luijten M, Maggi A (2011) Transgenic animal models in toxicology: historical perspectives and future outlook. Toxicol Sci 121:207–233
Brinkman EK, Chen T, Amendola M, van Steensel B (2014) Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42:e168
Brophy B, Smolenski G, Wheeler T, Wells D, L’Huillier P, Laible G (2003) Cloned transgenic cattle produce milk with higher levels of β-casein and κ-casein. Nat Biotechnol 21:157–162
Comizzoli P, Marquant-Le Guienne B, Heyman Y, Renard J (2000) Onset of the first S-phase is determined by a paternal effect during the G1-phase in bovine zygotes. Biol Reprod 62:1677–1684
Deng S, Kongpan L, Wang F, Ning L, Liu G, Zhao Y, Lian Z (2014) One-step generation of myostatin gene knockout sheep via the CRISPR/Cas9 system
Golberg A, Rubinsky B (2010) A statistical model for multidimensional irreversible electroporation cell death in tissue. Biomed Eng Online 9:13
Grobet L, Martin LJR, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J (1997) A deletion in the bovine myostatin gene causes the double–muscled phenotype in cattle. Nat Genet 17:71–74
Harrison RL, Byrne BJ, Tung L (1998) Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett 435:1–5
Heiser WC (2000) Optimizing electroporation conditions for the transformation of mammalian cells. Transcription factor protocols. Springer, pp. 117–134
Hirata M, Tanihara F, Wittayarat M, Hirano T, Nguyen NT, Le QA, Namula Z, Nii M, Otoi T (2019) Genome mutation after introduction of the gene editing by electroporation of Cas9 protein (GEEP) system in matured oocytes and putative zygotes. In Vitro Cell Dev Biol Anim:1–6
Hodges CA, Stice SL (2003) Generation of bovine transgenics using somatic cell nuclear transfer. Reprod Biol Endocrinol 1:81
Ikeda M, Matsuyama S, Akagi S, Ohkoshi K, Nakamura S, Minabe S, Kimura K, Hosoe M (2017) Correction of a disease mutation using CRISPR/Cas9-assisted genome editing in Japanese Black cattle. Sci Rep 7:17827
Isobe T, Ikebata Y, Onitsuka T, Do LT, Sato Y, Taniguchi M, Otoi T (2013) Cryopreservation for bovine embryos in serum-free freezing medium containing silk protein sericin. Cryobiology 67:184–187
Jordan ET, Collins M, Terefe J, Ugozzoli L, Rubio T (2008) Optimizing electroporation conditions in primary and other difficult-to-transfect cells. J Biomol Tech: JBT 19:328
Kambadur R, Sharma M, Smith TP, Bass JJ (1997) Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7:910–915
Luo J, Song Z, Yu S, Cui D, Wang B, Ding F, Li S, Dai Y, Li N (2014) Efficient generation of myostatin (MSTN) biallelic mutations in cattle using zinc finger nucleases. PLoS One 9:e95225
McPherron AC, Lee S-J (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci 94:12457–12461
Mori M, Otoi T, Suzuki T (2002) Correlation between the cell number and diameter in bovine embryos produced in vitro. Reprod Domest Anim 37:181–184
Niemann H, Kues WA (2003) Application of transgenesis in livestock for agriculture and biomedicine. Anim Reprod Sci 79:291–317
Nishio K, Tanihara F, Nguyen TV, Kunihara T, Nii M, Hirata M, Takemoto T, Otoi T (2018) Effects of voltage strength during electroporation on the development and quality of in vitro-produced porcine embryos. Reprod Domest Anim 53:313–318
Pherron A, Lawler A, Lee S (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387:83–90
Putri RI, Syamsiana IN (2010) Design of high voltage pulse generator for pasteurization by pulse electric field (PEF). Int J Comput Elect Eng 2:916
Ruan J, Xu J, Chen-Tsai RY, Li K (2017) Genome editing in livestock: are we ready for a revolution in animal breeding industry? Transgenic Res 26:715–726
Sosa MAG, De Gasperi R, Elder GA (2010) Animal transgenesis: an overview. Brain Struct Funct 214:91–109
Sun Z, Wang M, Han S, Ma S, Zou Z, Ding F, Li X, Li L, Tang B, Wang H (2018) Production of hypoallergenic milk from DNA-free beta-lactoglobulin (BLG) gene knockout cow using zinc-finger nucleases mRNA. Sci Rep 8:15430
Tanihara F, Takemoto T, Kitagawa E, Rao S, Do LTK, Onishi A, Yamashita Y, Kosugi C, Suzuki H, Sembon S (2016) Somatic cell reprogramming-free generation of genetically modified pigs. Sci Adv 2:e1600803
van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S (2011) Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update 17:620–627
Vilarino M, Rashid ST, Suchy FP, McNabb BR, Van Der Meulen T, Fine EJ, Ahsan S, Mursaliyev N, Sebastiano V, Diab SS (2017) CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep 7:17472
Wang K, Ouyang H, Xie Z, Yao C, Guo N, Li M, Jiao H, Pang D (2015a) Efficient generation of myostatin mutations in pigs using the CRISPR/Cas9 system. Sci Rep 5:16623
Wang T, Wei JJ, Sabatini DM, Lander ES (2014) Genetic screens in human cells using the CRISPR-Cas9 system. Science 343:80–84
Wang W, Zhang Y, Wang H (2017) Generating mouse models using zygote electroporation of nucleases (ZEN) technology with high efficiency and throughput. Zygotic Genome Activation: Methods and Protocols:219–230
Wang X, Yu H, Lei A, Zhou J, Zeng W, Zhu H, Dong Z, Niu Y, Shi B, Cai B (2015b) Generation of gene-modified goats targeting MSTN and FGF5 via zygote injection of CRISPR/Cas9 system. Sci Rep 5:13878
Wei J, Gaynor P, Cole S, Brophy B, Oback B, Laible G (2018) Developing the laboratory conditions for bovine zygote-mediated genome editing by electroporation. Proceedings of the World Congress on Genetics Applied to Livestock Production, 111118,
Acknowledgments
We thank the staff of the Meat Inspection Office of Tokushima Prefecture for supplying the bovine ovaries.
Funding
This study was supported in part by the JSPS KAKENHI grant numbers JP17H03938, JP17K19325, and JP18K12062. We acknowledge Tokushima University for their financial support of the Research Clusters program of Tokushima University (No. 1701001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Namula, Z., Wittayarat, M., Hirata, M. et al. Genome mutation after the introduction of the gene editing by electroporation of Cas9 protein (GEEP) system into bovine putative zygotes. In Vitro Cell.Dev.Biol.-Animal 55, 598–603 (2019). https://doi.org/10.1007/s11626-019-00385-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00385-w