Abstract
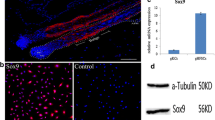
In our previous work, we found that the Inner Mongolia Arbas Cashmere goat hair follicle stem cells (gHFSCs) can be successfully differentiated into adipocyte, chondrocyte, and osteocyte lineages. In this study, we further examined the expression of the pluripotency and stemness markers Oct4, Nanog, Sox2, AKP, and TERT in gHFSCs by immunocytochemistry, flow cytometry, real-time PCR, and Western blot. Immunofluorescent staining showed that the gHFSCs were positive for all five markers. Fluorescence-activated cell sorting (FACS) further analyzed the positive expression of Oct4, Nanog, and Sox2 in the gHFSCs. Compared with Arbas Cashmere goat adipose-derived stem cells (gADSCs) at the mRNA expression level, Oct4 was relatively highly expressed in gHFSCs, 41.36 times of the gADSCs, and Nanog was 5.61, AKP was 2.74, and TERT was 2.10 times, respectively (p < 0.01). Western blot indicated that all markers are expressed at the protein level in the gHFSCs. When compared with gADSCs, using α-tubulin as a reference protein, gray intensity analysis showed that the expression of Oct4, Nanog, AKP, and TERT were, respectively, 5.94, 10.78, 1.33, and 1.39 times of gADSCs. Additionally, mRNA and protein expression of Sox2 were detected in the gHFSCs but not in the gADSCs. The protein expression pattern of these markers was consistent with the mRNA results.





Similar content being viewed by others
References
Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM (2005) Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. PNAS 102:5530–5534
Brett VJ, Rathjen J, Rathjen PD (2006) Transcriptional control of pluripotency: decisions in early development. Curr Opin Genet Dev 16:447–454
Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450:1230–1234
Chen X, Xu H, Yuan P, Fang F (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133:1106–1117
Cotsarelis G, Sun T-T, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61:1329–1337
de Lange T (2009) How telomeres solve the end-protection problem. Science 326:948–952
Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132:567–582
Jimenez F, Izeta A, Poblet E (2010) Morphometric analysis of the human scalp hair follicle: practical implications for the hair transplant surgeon and hair regeneration studies. Dermatol Surg 37:58–64
Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, Mii S, Amoh Y, Katsuoka K, Hoffman RM (2011) The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 10(5):830–839
Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov A (2007) Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 9:625–635
Niwa H, Masui S, Chambers I, Smith AG, Miyazaki J (2002) Phenotypic complementation establishes requirements for specific POU domain and generic transactivation function of Oct-3/4 in embryonic stem cells. Mol Cell Biol 22:1526–1536
Nowak JA, Fuchs E (2009) Isolation and culture of epithelial stem cells. Methods Mol Biol 482:215–232
O’Connor MD, Kardel MD, Iosfina I, Youssef D, Lu M, Li MM, Vercauteren S, Nagy A, Eaves CJ (2008) Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells 26:1109–1116
Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC (2006) Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 116:249–260
Podlevsky JD, Chen JJL (2012) It all comes together at the ends: telomerase structure, function, and biogenesis. Mutat Res 730:3–11
Purba TS, Haslam IS, Poblet E, Jiménez F, Gandarillas A, Izeta A, Paus R (2014) Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays 36:513–525
Ren Y, Wu H, Wang X, Xue N, Liang H, Liu D (2014) Analysis of the stem cell characteristics of adult stem cells from Arbas white Cashmere goat. BBRC 448:121–128
Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H (2006) Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s C T difference” formula. J Mol Med 84:901–910
Shay JW, Wright WE (2010) Telomeres and telomerase in normal and cancer stem cells. FEBS Lett 584:3819–3825
Štefková K, Procházková J, Pacherník J (2015) Alkaline phosphatase in stem cells. Stem Cells Int 2015:628368
Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM (2000) Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102:451–461
Trounson A, Thakar RG, Lomax G, Gibbons D (2011) Clinical trials of stem cell therapies. BMC Med 9:52
Yamanaka S, Takahashi K (2006) Induction of pluripotent stem cells from mouse fibroblast cultures. Tanpakushitsu Kakusan Koso 51:2346–2351
Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y (2015) From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 14(14):2362–2366
Young RA (2011) Control of the embryonic stem cell state. Cell 144:940–954
Yu HFD, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X (2006) Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol 168:1879–1888
Acknowledgments
This work was supported by a grant from the Key Special Projects in Breeding New Varieties of Genetically Engineered Organisms (2014ZX08008002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Editor: Tetsuji Okamoto
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3081 kb)
Rights and permissions
About this article
Cite this article
He, N., Dong, Z., Zhu, B. et al. Expression of pluripotency markers in Arbas Cashmere goat hair follicle stem cells. In Vitro Cell.Dev.Biol.-Animal 52, 782–788 (2016). https://doi.org/10.1007/s11626-016-0023-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0023-3