Abstract
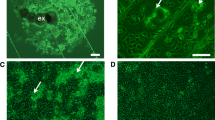
We established a continuous cell line from the embryo of the mosquito Culex tritaeniorhynchus Giles (Diptera: Culicidae), a known major vector of the Japanese encephalitis virus (family Flaviviridae, genus Flavivirus) in Asia. The cell line, designated NIID-CTR, was serially subcultured in VP-12 medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS). It continued to grow for more than 60 passages over a 750-d period. The NIID-CTR cell line mainly comprised two morphologically distinct types of cells with adhesive properties: spindle-shaped and round cells. Most of the NIID-CTR cells at the 45th passage were diploid (2n = 6). The growth kinetics of the NIID-CTR cells was significantly affected by the FBS concentration in the medium. The population doubling time of the NIID-CTR cells was 20 h in the presence of 10 % FBS and 76 h in its absence. The DNA sequence of the mitochondrial cytochrome oxidase I gene confirmed that the NIID-CTR cell line was derived from C. tritaeniorhynchus. The cells were highly susceptible to Japanese encephalitis and Dengue viruses, thus providing a valuable tool for the study of mosquito-borne flaviviruses.






Similar content being viewed by others
References
Athawale S. S.; Sudeep A. B.; Barde P. V.; Jadi R.; Pant U.; Mishra A. C.; Mourya D. T. A new cell line from the embryonic tissues of Culex tritaeniorhynchus and its susceptibility to certain flaviviruses. Acta Virol. 46(4): 237–240; 2002.
Brackney D. E.; Beane J. E.; Ebel G. D. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathog. 5(7): e1000502; 2009.
Brackney D. E.; Scott J. C.; Sagawa F.; Woodward J. E.; Miller N. A.; Schilkey F. D.; Mudge J.; Wilusz J.; Olson K. E.; Blair C. D.; Ebel G. D. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl. Trop. Dis. 4(10): e856; 2010.
Breland O. P. Studies on the chromosomes of mosquitoes. Ann. Entomol. Soc. Am. 54(3): 360–375; 1961.
Campbell C. L.; Keene K. M.; Brackney D. E.; Olson K. E.; Blair C. D.; Wilusz J.; Foy B. D. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 8: 47; 2008.
Chao J.; Ball G. H. A comparison of amino acid utilization by cell lines of Culex tarsalis and Culex pipiens. In: Kurstak E.; Maramorosch K. (eds) Invertebrate tissue culture: applications in medicine, biology and agriculture. Academic Press, New York, pp 263–266; 1976.
Chen Y. Y.; Fan Y. C.; Tu W. C.; Chang R. Y.; Shih C. C.; Lu I. H.; Chien M. S.; Lee W. C.; Chen T. H.; Chang G. J.; Chiou S. S. Japanese encephalitis virus genotype replacement; Taiwan; 2009–2010. Emerg Infect. Dis. 17(12): 2354–2356; 2011.
Chotkowski H. L.; Ciota A. T.; Jia Y.; Puig-Basagoiti F.; Kramer L. D.; Shi P. Y.; Glaser R. L. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology 377(1): 197–206; 2008.
Cirimotich C. M.; Scott J. C.; Phillips A. T.; Geiss B. J.; Olson K. E. Suppression of RNA interference increases alphavirus replication and virus-associated mortality in Aedes aegypti mosquitoes. BMC Microbiol. 9: 49; 2009.
Cook S.; Lien N. G.; McAlister E.; Harbach R. E. Bothaella manhi, a new species of tribe Aedini (Diptera: Culicidae) from the Cuc Phuong National Park of Vietnam based on morphology and DNA sequence. Zootaxa 2661: 33–46; 2010.
Dimopoulos G.; Richman A.; Müller H. M.; Kafatos F. C. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc. Nat. Acad. Sci. U. S. A. 94(21): 11508–11513; 1997.
Erlanger T. E.; Weiss S.; Keiser J.; Utzinger J.; Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 15(1): 1–7; 2009.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4): 783–791; 1985.
Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences. University of Washington, Seattle; 2005.
Freshney R. I. Culture of animal cells (5th edition): a manual of basic technique. John Wiley & Sons, Inc., Hoboken; 2005.
Giulivi C.; Ross-Inta C.; Horton A. A.; Luckhart S. Metabolic pathways in Anopheles stephensi mitochondria. Biochem. J. 415(2): 309–316; 2008.
Grace T. D. C. Establishment of a line of mosquito (Aedes aegypti L.) cells grown in vitro. Nature 211(5047): 366–367; 1966.
Hoshino K.; Isawa H.; Tsuda Y.; Kobayashi M. Laboratory colonization of Aedes japonicus japonicus (Diptera: Culicidae) collected in Narita, Japan and the biological properties of the established colony. Jpn. J. Infect. Dis. 63(6): 401–404; 2010.
Hsu, S. H. Growth of arboviruses in arthropod cell cultures: comparative studies. I. Preliminary observations on growth of arboviruses in a newly established line of mosquito cell (Culex quinquefasciatus Say). In : Welss, E. (ed.) Curr. Top. Microbiol. Immunol. 55. Springer, Berlin Heidelberg New York, pp 140–148; 1971.
Hsu S. H.; Li S. Y.; Cross J. H. A cell line derived from ovarian tissue of Culex tritaeniorhynchus summorosus Dyar. J. Med. Entomol. 9(1): 86–91; 1972.
Hsu S. H.; Mao W. H.; Cross J. H. Establishment of a line of cells derived from ovarian tissue of Culex quinquefasciatus Say. J. Med. Entomol. 7(6): 703–707; 1970.
Hsu S. H.; Wang B. T.; Huang M. H.; Wong W. J.; Cross J. H. Growth of Japanese encephalitis virus in Culex tritaeniorhynchus cell cultures. Am.J.Trop. Med. Hyg. 24(5): 881–888; 1975.
Hughes G. L.; Ren X.; Ramirez J. L.; Sakamoto J. M.; Bailey J. A.; Jedlicka A. E.; Rasgon J. L. Wolbachia infections in Anopheles gambiae cells: transcriptomic characterization of a novel host–symbiont interaction. PLoS Pathog. 7(2): e1001296; 2011.
Igarashi A. Isolation of a Singh’s Aedes albopictus cell colne sensitive to dengue and chikungunya viruses. J. Gen. Virol. 40(3): 531–544; 1978.
Keene K. M.; Foy B. D.; Sanchez-Vargas I.; Beaty B. J.; Blair C. D.; Olson K. E. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Nat. Acad. Sci. U. S. A. 101(49): 17240–17245; 2004.
Kimura M. A simple method for estimating evolutionary rate of base substitution through comparative study of nucleotide sequences. J. Mol. Evolution 16(2): 111–120; 1980.
Kitamura S. Establishment of cell line from Culex mosquito. Kobe J. Med. Sci. 16(1): 41–50; 1970.
Lan Q.; Fallon A. M. Small heat shock proteins distinguish between two mosquito species and confirm identity of their cell lines. Am.J.Trop. Med. Hyg. 43(6): 669–676; 1990.
Larkin M. A.; Blackshields G.; Brown N. P.; Chenna R.; McGettigan P. A.; McWilliam H.; Valentin F.; Wallace I. M.; Wilm A.; Lopez R.; Thompson J. D.; Gibson T. J.; Higgins D. G. Clustal W and Clustal X version 2.0. Bioinformatics 23(21): 2947–2948; 2007.
Li M. H.; Fu S. H.; Chen W. X.; Wang H. Y.; Guo Y. H.; Liu Q. Y.; Li Y. X.; Luo H. M.; Da W.; Duo Ji D. Z.; Ye X. M.; Liang G. D. Genotype V Japanese encephalitis virus is emerging. PLoS Negl. Trop. Dis. 5(7): e1231; 2011.
Maharaj P. D.; Anishchenko M.; Langevin S. A.; Fang Y.; Reisen W. K.; Brault A. C. Structural gene (prME) chimeras of St. Louis encephalitis virus and West Nile virus exhibit altered in vitro cytopathic and growth phenotypes. J. Gen. Virol. 93(1): 39–49; 2011.
Main O. M.; Hardy J. L.; Reeves W. C. Growth of arboviruses and other viruses in a continuous line of Culex tarsalis cells. J. Med. Entomol. 14(1): 107–112; 1977.
Mangada M. N.; Takegami T. Molecular characterization of the Japanese encephalitis virus representative immunotype strain JaGAr 01. Virus Res. 59(1): 101–112; 1999.
Marhoul Z.; Pudney M. A mosquito cell line (MOS. 55) from Anopheles gambiae larva. Trans. R. Soc. Trop. Med. Hyg. 66(1): 183–184; 1972.
Mitsuhashi J. List of reported continuous invertebrate cell lines. In: Mitsuhashi J. (ed) Invertebrate tissue culture methods. Springer, Japan, pp 421–430; 2002.
Mitsuhashi J.; Maramorosch K. Leafhopper tissue culture: embryonic, nymphal, and imaginal tissues from aseptic insects. Contrib. Boyce Thompson Inst. 22(8): 435–460; 1964.
Müller H. M.; Dimopoulos G.; Blass C.; Kafatos F. C. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 274(17): 11727–11735; 1999.
Myles K. M.; Wiley M. R.; Morazzani E. M.; Adelman Z. N. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proc. Nat. Acad. Sci. U. S. A. 105(50): 19938–19943; 2008.
Nerome R.; Tajima S.; Takasaki T.; Yoshida T.; Kotaki A.; Lim C. K.; Ito M.; Sugiyama A.; Yamauchi A.; Yano T.; Kameyama T.; Morishita I.; Kuwayama M.; Ogawa T.; Sahara K.; Ikegaya A.; Kanda M.; Hosoya Y.; Itokazu K.; Onishi H.; Chiya S.; Yoshida Y.; Tabei Y.; Katsuki K.; Tabata K.; Harada S.; Kurane I. Molecular epidemiological analyses of Japanese encephalitis virus isolates from swine in Japan from 2002 to 2004. J. Gen. Virol. 88(10): 2762–2768; 2007.
Nga P. T.; del Carmen Parquet M.; Cuong V. D.; Ma S. P.; Hasebe F.; Inoue S.; Makino Y.; Takagi M.; Nam V. S.; Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J. Gen. Virol. 85(6): 1625–1631; 2004.
Oelofsen M. J.; Gericke A.; Smith M. S.; van der Linde T. C. Establishment and characterization of a cell line from the mosquito Culex (Culex) theileri (Diptera: Culicidae) and its susceptibility to infection with arboviruses. J. Med. Entomol. 27(6): 939–944; 1990.
Page, R. D. M. TreeView Version 1. 6. 6.. http://taxonomy.zoology.gla.ac.uk/rod/treeview.html; 2001.
Pan X. L.; Liu H.; Wang H. Y.; Fu S. H.; Liu H. Z.; Zhang H. L.; Li M. H.; Gao X. Y.; Wang J. L.; Sun X. H.; Lu X. J.; Zhai Y. G.; Meng W. S.; He Y.; Wang H. Q.; Han N.; Wei B.; Wu Y. G.; Feng Y.; Yang D. J.; Wang L. H.; Tang Q.; Xia G.; Kurane I.; Rayner S.; Liang G. D. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J. Virol. 85(19): 9847–9853; 2011.
Pant U.; Banerjee K.; Athawale S. A.; Dhanda V. Susceptibility of Culex bitaeniorhynchus cell line to some arboviruses. Indian J. Med. Res. 76: 789–794; 1982.
Pant U.; Dhanda V. Establishment of a cell line from Culex bitaeniorhynchus. J. Tissue Culture Methods 6(2): 61–63; 1980.
Peleg J. Growth of arboviruses in primary tissue culture of Aedes aegypti embryos. Am.J.Trop. Med. Hyg. 17(2): 219–223; 1968.
Pudney M.; Varma M. G. Anopheles stephensi var. mysorenis: establishment of a larval cell line (Mos. 43). Exp. Parasitol. 29(1): 7–12; 1971.
Saitou N.; Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4(4): 406–425; 1987.
Sánchez-Vargas I.; Scott J. C.; Poole-Smith B. K.; Franz A. W.; Barbosa-Solomieu V.; Wilusz J.; Olson K. E.; Blair C. D. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito’s RNA interference pathway. PLoS Pathog. 5(2): e1000299; 2009.
Scott J. C.; Brackney D. E.; Campbell C. L.; Bondu-Hawkins V.; Hjelle B.; Ebel G. D.; Olson K. E.; Blair C. D. Comparison of dengue virus type 2-specific small RNAs from RNA interference-competent and -incompetent mosquito cells. PLoS Negl. Trop. Dis. 4(10): e848; 2010.
Singh K. R. P. Cell cultures derived from larvae of Aedes albopictus (Skuse) and Aedes aegypti (L.). Curr. Sci. 36(19): 506–508; 1967.
Siu R. W.; Fragkoudis R.; Simmonds P.; Donald C. L.; Chase-Topping M. E.; Barry G.; Attarzadeh-Yazdi G.; Rodriguez-Andres J.; Nash A. A.; Merits A.; Fazakerley J. K.; Kohl A. Antiviral RNA interference responses induced by Semliki Forest virus infection of mosquito cells: characterization, origin, and frequency-dependent functions of virus-derived small interfering RNAs. J. Virol. 85(6): 2907–2917; 2011.
Solomon T.; Ni H.; Beasley D. W.; Ekkelenkamp M.; Cardosa M. J.; Barrett A. D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 77(5): 3091–3098; 2003.
Tajima S.; Nukui Y.; Ito M.; Takasaki T.; Kurane I. Nineteen nucleotides in the variable region of 3′ non-translated region are dispensable for the replication of dengue type 1 virus in vitro. Virus Res. 116: 38–44; 2006.
Takhampunya R.; Kim H. C.; Tippayachai B.; Kengluecha A.; Klein T. A.; Lee W. J.; Grieco J.; Evans B. P. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virology J. 8: 449; 2011.
Tesh R. B. Establishment of two cell lines from the mosquito Toxorhynchites amboinensis (Diptera: Culicidae) and their susceptibility to infection with arboviruses. J. Med. Entomol. 17(4): 338–343; 1980.
van den Hurk A. F.; Ritchie S. A.; Mackenzie J. S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 54: 17–35; 2009.
Vanlandingham D. L.; Hong C.; Klingler K.; Tsetsarkin K.; McElroy K. L.; Powers A. M.; Lehane M. J.; Higgs S. Differential infectivities of o’nyong-nyong and chikungunya virus isolates in Anopheles gambiae and Aedes aegypti mosquitoes. Am.J.Trop. Med. Hyg. 72(5): 616–621; 2005.
Varma M. G. R.; Pudney M. The growth and serial passage of cell lines from Aedes aegypti (L.) larvae in different media. J. Med. Entom. 6(4): 432–439; 1969.
World Health Organization. http://www.who.int/mediacentre/factsheets/fs117/en/index.html; 2009.
Aknowledgements
This work was partially supported by a grant from Japanese Ministry of Health, Labor and Welfare (21-Shinko-Ippan-005), a Grant-in-aids from the Japan Society for the Promotion of Science (Scientific Research C, no. 22590387), and a grant from Japanese Ministry of the Environment (Global Environment Research Fund, S-8).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Kuwata, R., Hoshino, K., Isawa, H. et al. Establishment and characterization of a cell line from the mosquito Culex tritaeniorhynchus (Diptera: Culicidae). In Vitro Cell.Dev.Biol.-Animal 48, 369–376 (2012). https://doi.org/10.1007/s11626-012-9520-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9520-1