Abstract
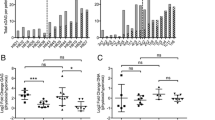
Previous studies have demonstrated that oxygen environment is an important determinate factor of cell phenotypes and differentiation, although factors which affect pericellular oxygen concentration (POC) in murine chondrogenic cell culture remain unidentified. Oxygen concentrations in vivo were measured in rabbit musculoskeletal tissues, which were by far hypoxic compared to 20% O2 (ranging from 2.29 ± 1.16 to 4.36 ± 0.51%). Oxygen concentrations in murine chondrogenic cell (C3H10T1/2) culture medium were monitored in different oxygen concentrations (20% or 5%) in the incubator and in different medium volumes (3,700 or 7,400 μl) within 25-cm2 flasks. Chondrogenic differentiation was assessed by glycosaminoglycan production with quantitative evaluation of Alcian blue staining in 12-well culture dishes. Expression of chondrogenic genes, aggrecan, and type II collagen α1, was examined by quantitative real-time polymerase chain reaction. Oxygen concentrations in medium decreased accordingly with the depth from medium surface, and POC at Day 6 was 18.99 ± 0.81% in 3,700-μl medium (1,480-μm depth) and 13.26 ± 0.23% in 7,400-μl medium (2,960-μm depth) at 20% O2 in the incubator, which was 4.96 ± 0.08% (1,480-μm depth) and 2.83 ± 0.42% (2,960-μm depth) at 5% O2, respectively. The differences of POC compared by medium volume were statistically significant (p = 0.0003 at 20% and p = 0.001 at 5%). Glycosaminoglycan production and aggrecan gene expression were most promoted when cultured in moderately low POC, 1,000 μl (2,960-μm depth) at 20% O2 and 500 μl (1,480-μm depth) at 5% O2 in 12-well culture dishes. We demonstrate that medium volume and oxygen concentration in the incubator affect not only POC but also chondrogenic differentiation.





Similar content being viewed by others
References
Asahina I.; Sampath T. K.; Hauschka P. V. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp. Cell Res. 222: 38–47; 1996.
Bassett C. A.; Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature 190: 460–461; 1961.
Brighton C. T.; Wang W.; Clark C. C. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J. Bone Joint Surg. Am. 90: 833–848; 2008.
Chapman J. D.; Sturrock J.; Boag J. W.; Crookall J. O. Factors affecting the oxygen tension around cells growing in plastic Petri dishes. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 17: 305–328; 1970.
Chen L.; Fink T.; Ebbesen P.; Zachar V. Hypoxic treatment inhibits insulin-induced chondrogenesis of ATDC5 cells despite upregulation of DEC1. Connect. Tissue Res. 47: 119–123; 2006.
Cipolleschi M. G.; Dello Sbarba P.; Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82: 2031–2037; 1993.
Ebbesen P.; Eckardt K. U.; Ciampor F.; Pettersen E. O. Linking measured intercellular oxygen concentration to human cell functions. Acta Oncol. 43: 598–600; 2004.
Elder B. D.; Athanasiou K. A. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng. Part B Rev. 15: 43–53; 2009.
Elder S. H.; Fulzele K. S.; McCulley W. R. Cyclic hydrostatic compression stimulates chondroinduction of C3H/10T1/2 cells. Biomech. Model. Mechanobiol. 3: 141–146; 2005.
Evers B.; Odemis V.; Gerngross H. Oxygen partial pressure in the anterior tibial muscle during and after knee surgery with tourniquet control. Adv. Exp. Med. Biol. 428: 317–325; 1997.
Haas A. R.; Tuan R. S. Murine C3H10T1/2 multipotential cells as an in vitro model of mesenchymal chondrogenesis. Methods Mol. Biol. 137: 383–389; 2000.
Hirao M.; Tamai N.; Tsumaki N.; Yoshikawa H.; Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J. Biol. Chem. 281: 31079–31092; 2006.
Hopfl G.; Ogunshola O.; Gassmann M. HIFs and tumors—causes and consequences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286: R608–R623; 2004.
Kiaer T.; Gronlund J.; Sorensen K. H. Subchondral pO2, pCO2, pressure, pH, and lactate in human osteoarthritis of the hip. Clin. Orthop. Relat. Res. 229: 149–155; 1988.
Kofoed H.; Sjontoft E.; Siemssen S. O.; Olesen H. P. Bone marrow circulation after osteotomy. Blood flow, pO2, pCO2, and pressure studied in dogs. Acta Orthop. Scand. 56: 400–403; 1985.
Lee C. C.; Ye F.; Tarantal A. F. Comparison of growth and differentiation of fetal and adult rhesus monkey mesenchymal stem cells. Stem Cells Dev. 15: 209–220; 2006.
Lim Y. B.; Kang S. S.; Park T. K.; Lee Y. S.; Chun J. S.; Sonn J. K. Disruption of actin cytoskeleton induces chondrogenesis of mesenchymal cells by activating protein kinase C-alpha signaling. Biochem. Biophys. Res. Commun. 273: 609–613; 2000.
Malladi P.; Xu Y.; Chiou M.; Giaccia A. J.; Longaker M. T. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 290: C1139–C1146; 2006.
Mamchaoui K.; Saumon G. A method for measuring the oxygen consumption of intact cell monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 278: L858–L863; 2000.
Metzen E.; Wolff M.; Fandrey J.; Jelkmann W. Pericellular pO2 and O2 consumption in monolayer cell cultures. Respir. Physiol. 100: 101–106; 1995.
Pettersen E. O.; Larsen L. H.; Ramsing N. B.; Ebbesen P. Pericellular oxygen depletion during ordinary tissue culturing, measured with oxygen microsensors. Cell Prolif. 38: 257–267; 2005.
Pilgaard L.; Lund P.; Duroux M.; Fink T.; Ulrich-Vinther M.; Soballe K.; Zachar V. Effect of oxygen concentration, culture format and donor variability on in vitro chondrogenesis of human adipose tissue-derived stem cells. Regen. Med. 4: 539–548; 2009.
Richter A.; Sanford K. K.; Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J. Natl. Cancer Inst. 49: 1705–1712; 1972.
Shukunami C.; Ohta Y.; Sakuda M.; Hiraki Y. Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp. Cell Res. 241: 1–11; 1998.
Wagner D. R.; Lindsey D. P.; Li K. W.; Tummala P.; Chandran S. E.; Smith R. L.; Longaker M. T.; Carter D. R.; Beaupre G. S. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann. Biomed. Eng. 36: 813–820; 2008.
Wolff M.; Fandrey J.; Jelkmann W. Microelectrode measurements of pericellular pO2 in erythropoietin-producing human hepatoma cell cultures. Am. J. Physiol. 265: C1266–C1270; 1993.
Acknowledgments
We would like to thank Mari Shinkawa for her excellent technical assistances, and we also would like to thank Osteopharma for supplying recombinant human BMP-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Oze, H., Hirao, M., Ebina, K. et al. Impact of medium volume and oxygen concentration in the incubator on pericellular oxygen concentration and differentiation of murine chondrogenic cell culture. In Vitro Cell.Dev.Biol.-Animal 48, 123–130 (2012). https://doi.org/10.1007/s11626-011-9479-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-011-9479-3