Abstract
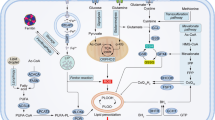
Colon adenocarcinoma is one of the most common fatal malignancies in Western countries. Progression of this cancer is dependent on tumor microenvironmental signaling molecules such as transforming growth factor-β (TGF-β) or acetylcholine (ACh). The present study was conducted to assess the influence of recombinant human transforming growth factor (rhTGF)-β1 or ACh on nitric oxide (NO) and interleukin-1β (IL-1β) secretion by three human colon adenocarcinoma cell lines: HT29, LS180, and SW948, derived from different grade tumors (Duke’s stage). The cells were cultured in 2D and 3D (spheroids) conditions. Colon carcinoma cells exhibited different sensitivities to rhTGF-β1 or ACh dependent on the tumor grade and the culture model. ACh exhibited significant inhibitory effects towards NO, endothelial nitric oxide synthase (eNOS), and IL-1β secretion especially by tumor cells derived form Duke’s C stage of colon carcinoma. rhTGF-β1 also decreased NO, IL-1β, and eNOS expression, but its effect was lower than that observed after the administration of ACh. The inhibition of NO and IL-1β production was more striking in 3D tumor spheroids than in 2D culture monolayers. Taken together, the TGF-β1–ACh axis may regulate colon carcinoma progression and metastasis by altering NO secretion and influence inflammatory responses by modulating IL-1β production.





Similar content being viewed by others
References
Apte R. N.; Dotan S.; Elkabets M.; White M. R.; Reich E.; Carmi Y.; Song X.; Dvozkin T.; Krelin Y.; Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor–host interactions. Cancer Metastasis Rev. 25: 387–408; 2006.
Ashwood P.; Harvey R.; Verjee T.; Wolstencroft R.; Thompson R. P. H.; Powell J. J. Competition between IL-1, IL-1ra and TGF-β1 modulates the response of the ELA4.NOB-1/CTLL bioassay: implications for clinical investigations. Inflamm. Res. 53: 60–65; 2004.
Basu S.; Dasgupta P. S. Decreased dopamine receptor expression and its second-messenger camp in malignant human colon tissue. Dig. Dis. Sci. 44: 916–921; 1999.
Bellone G.; Carbone A.; Tibaudi D.; Mauri F.; Ferrero I.; Smirne C.; Suman F.; Rivetti C.; Migliaretti G.; Camandona M.; Palestro G.; Emanuelli G.; Rodeck U. Differential expression of transforming growth factors-β1, -β2 and -β3 in human colon carcinoma. Eur. J. Cancer 37: 224–233; 2001.
Bonavida B.; Khineche S.; Huerta-Yepez S.; Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resist. Updat. 9: 157–173; 2006.
Cheng K.; Zimniak P.; Raufman J.-P. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 63: 6744–6750; 2003.
Español A.; Eiján A. M.; Mazzoni E.; Davel L.; Jasnis M. A.; Sacerdote De Lustig E.; Sales M. E. Nitric oxide synthase, arginase and cyclooxygenase are involved in muscarinic receptor activation in different murine mammary adenocarcinoma cell lines. Int. J. Mol. Med. 9: 651–657; 2002.
Eutamene H.; Theodorou V.; Fioramonti J.; Bueno L. Implicatin of NK1 and NK2 receptors in rat colonic hypersecretion induced by interleukin 1β: role of nitric oxide. Gastroenterology 109: 483–489; 1995.
Galizia G.; Orditura M.; Romano C.; Lieto E.; Castellano P.; Pelosio L.; Imperatore V.; Catalano G.; Pignatelli C.; De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin. Immunol. 102: 169–178; 2002.
Greco E.; Basso D.; Fogar P.; Mazza S.; Navaglia F.; Zambon C. F.; Falda A.; Pedrazzoli S.; Ancona E.; Plebani M. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1β not by transforming growth factor-β1. Int. J. Biol. Markers 20: 235–241; 2005.
Hill M. J. Molecular and clinical risk markers in colon cancer trials. Eur. J. Cancer 36: 1288–1291; 2000.
Joseph J.; Niggemann B.; Zaenker K. S.; Entschladen F. Anandamide is a endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol. Immunother. 53: 723–728; 2004.
Lala P. K.; Chakraborty Ch. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 3: 149–156; 2001.
Lewis A. M.; Varghese S.; Xu H.; Alexander H. R. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J. Translat. Med. 4: 48–59; 2006.
Li F.; Cao J.; Townsend C. M. Jr.; Ko T. C. TGF-β signalling in colon cancer cells. World J. Surg. 29: 306–311; 2005.
Lohm S.; Peduto-Eberl L.; Lagadec P.; Renggli-Zulliger N.; Dudler J.; Jeannin J.-F.; Juillerat-Jeanneret L. Evaluation of the interaction between TGF-β and nitric oxide in the mechanisms of progression of colon carcinoma. Clin. Exp. Metastasis 22: 341–349; 2005.
Moilanen E.; Vapaatalo H. Nitric oxide in inflammation and immune response. Ann. Med. 27: 359–367; 1995.
Paduch R.; Walter-Croneck A.; Zdzisińska B.; Szuster-Ciesielska A.; Kandefer-Szerszeń M. Role of reactive oxygen species (ROS), metalloproteinase-2 (MMP-2) and interleukin-6 (IL-6) in direct interactions between tumour cell spheroids and endothelial cell monolayer. Cell Biol. Int. 29: 497–505; 2005.
Quyyumi A. A.; Dakak N.; Mulcahy D.; Andrews N. P.; Husain S.; Panza J. A.; Cannon R. O. III Nitric oxide activity in the atherosclerotic human coronary circulation. J. Am. Coll. Cardiol. 29: 308–317; 1997.
Rao A. A.; Sridhar G. R.; Das U. N. Elevated butyrylcholinesterase and acetylcholineesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med. Hypotheses 69: 1272–1276; 2007.
Rao Ch. V. Nitric oxide signalling in colon cancer chemoprevention. Mutat. Res. 555: 107–119; 2004.
Ruscetti F. W.; Dubois C. M.; Jacobsen S. E.; Keller J. R. Transforming growth factor β and interleukin-1: a paradigm for opposing regulation of haemopoiesis. Baillieres Clin. Haematol. 5: 703–721; 1992.
Shen Z.-X. Rationale for diagnosing deficiency of ChEs and for applying exogenous HuChEs to the treatment of diseases. Med. Hypotheses 70: 43–51; 2008.
Song P.; Sekhon H. S.; Proskocil B.; Blusztajn J. K.; Mark G. P.; Spindel E. R. Synthesis of acetylcholine by lung cancer. Life Sci. 72: 2159–2168; 2003.
Tozer G. M.; Prise V. E.; Bell K. M.; Dennis M. F.; Stratford M. R.; Chaplin D. J. Reduced capacity of tumour blood vessels to produce endothelium-derived relaxing factor: significance for blood flow modification. Br. J. Cancer 74: 1955–1960; 1996.
Tsushima H.; Kawata S.; Tamura S.; Ito N.; Shirai Y.; Kiso S.; Imai Y.; Shimomukai H.; Nomura Y.; Matsuda Y.; Matsuzawa Y. High levels of transforming growth factor β1 in patients with colorectal cancer: association with disease progression. Gastroenterology 110: 375–382; 1996.
Ukegawa J.-I.; Takeuchi Y.; Kusayanagi S.; Mitamura K. Growth-promoting effect of muscarinic acatylcholine receptors in colon cancer cells. J. Cancer Res. Clin. Oncol. 129: 272–278; 2003.
Yeh R. K.; Chen J.; Williams J. L.; Baluch M.; Hundley T. R.; Rosenbaum R. E.; Kalala S.; Traganos F.; Benardini F.; del Soldato P.; Kashfi K.; Rigas B. NO-donating nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: a general pharmacological property? Biochem. Pharmacol. 67: 2197–2205; 2004.
Yudoh K.; Matsui H.; Tsuji H. Nitric oxide induced by tumor cells activates tumor cell adhesion to endothelial cells and permeability of the endothelium in vitro. Clin. Exp. Metastasis 15: 557–567; 1997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Paduch, R., Kandefer-Szerszeń, M. Transforming growth factor-β1 (TGF-β1) and acetylcholine (ACh) alter nitric oxide (NO) and interleukin-1β (IL-1β) secretion in human colon adenocarcinoma cells. In Vitro Cell.Dev.Biol.-Animal 45, 543–550 (2009). https://doi.org/10.1007/s11626-009-9220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-009-9220-7