Abstract
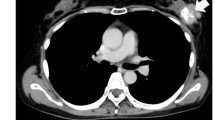
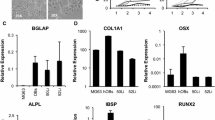
Osteosarcoma is the most common form of primary bone cancer. In this study, we established a human osteosarcoma cell line (OS 99-1) from a highly aggressive primary tumor. G-banding karyotype analysis demonstrated a large number of clonal abnormalities, as well as extensive intercellular heterogeneity. Through the use of immunologic, molecular, and biochemical analyses, we characterized protein and gene expression profiles confirming the osteogenic nature of the cells. Further evaluation indicated that OS 99-1 cells maintain the capacity to differentiate in an in vitro mineralization assay as well as form tumors in the in vivo chicken embryo model. This cell line provides a useful tool to investigate the molecular mechanisms contributing to osteosarcoma and may have the potential to serve as a culture system for studies involving bone physiology.





Similar content being viewed by others
Abbreviations
- ALP:
-
tissue nonspecific alkaline phosphatase
- ARS:
-
Alizarin Red S
- BSP:
-
bone sialoprotein
- LOH:
-
loss of heterogeneity
- MTT:
-
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- OC:
-
osteocalcin
- OS:
-
osteosarcoma
- PVDF:
-
polyvinylidene fluoride
- qRT-PCR:
-
quantitative real-time reverse transcriptase PCR
References
Ballester M.; Castello A. et al. Real-time quantitative PCR-based system for determining transgene copy number in transgenic animals. Biotechniques 37: 610–613; 2004.
Belchis D.A. and Gocke C.D. Alterations in the RB, p16, and cyclin D1 cell cycle control pathway in osteosarcomas. Pediatr. Pathol. Mol. Med. 19: 377–389; 2000.
Biegel J. A.; Womer R.B. et al. Complex karyotypes in a series of pediatric osteosarcomas. Cancer Genet. Cytogenet. 38: 89–100; 1989.
Bilbe G.; Roberts G. et al. PCR phenotyping of cytokines, growth factors and their receptors and bone matrix proteins in human osteoblast-like cell lines. Bone 19: 437–445; 1996.
Boehm A.K.; Neff, J.R. et al. Cytogenetic findings in 36 osteosarcoma specimens and a review of the literature. Pediatr. Pathol. Mol. Med. 19: 359–376; 2000.
Bridge J.; Nelson M. et al. Cytogenetic findings in 73 osteosarcoma specimens and a review of the literature. Cancer Genet. Cytogenet. 95: 74–87; 1997.
Chauveinc L.; Mosseri V. et al. Osteosarcoma following retinoblastoma: age at onset and latency period. Ophthalmic Genet. 22: 77–88; 2001.
Fisher L.W.; Stubbs J.T. et al. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop. Scand. Suppl. 266: 62–65; 1995.
Fletcher J.A.; Gebhardt M.C. et al. Cytogenetic aberrations in osteosarcomas: nonrandom deletions, rings, and double-minute chromosomes. Cancer Genet Cytogenet 77: 81–88; 1994.
Gillette J.M.; Nielsen-Preiss S.M. The role of annexin 2 in osteoblastic mineralization. J. Cell Sci. 117: 441–449; 2004.
Jacks T.; Remington L. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4: 1–7; 1994.
Jensh R.P.; Brent R.L. Rapid schedules for KOH clearing and Alizarin red S staining of fetal rat bone. Stain. Technol. 41: 179–183; 1966.
Kim S.; Choi J. et al. Imaging findings of extrapulmonary metastases of osteosarcoma. Clin Imaging 284: 291–230; 2004.
Kitchin F.D.; Ellsworth R.M. Pleiotropic effects of the gene for retinoblastoma. J. Med. Genet. 11: 244–246; 1974.
Lian J.; Stein G et al. Bone formation: osteoblast lineage cells, growth factors, matrix proteins, and the mineralization process. In: FavusM. (ed) Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Lippincott Williams & Wilkins, Philadelphia, pp 14–29; 1999.
Lim G., Karaskova J. et al. Combined spectral karyotyping, multicolor banding, and microarray comparative genomic hybridization analysis provides a detailed characterization of complex structural chromosomal rearrangement associated with gene amplification in the osteosarcoma cell line MG-63. Cancer Genet. Cytogenet. 153: 158–164; 2004.
Lui F., Malaval L. et al. The mature osteoblast phenotype is characterized by extensive plasticity. Exp. Cell. Res. 232: 97–105; 1997.
Marina N.M., Pratt C.B. et al. Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer 70: 2722–2727; 1992.
Masuda H., Miller C. et al. Rearrangement of the p53 gene in human osteogenic sarcomas.”. Proc. Natl. Acad. Sci. USA 84: 7716–7719; 1987.
Mertens F., Mandahl N. et al. Cytogenetic findings in 33 osteosarcomas. Int. J. Cancer 55: 44–50; 1993.
Meyers P.A., Heller G. et al. Chemotherapy for nonmetastatic osteogenic sarcoma: The Memorial Sloan–Kettering experience. J. Clin. Oncol. 10: 5–15; 1992.
Meyers P.A., Heller G. et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J. Clin. Oncol. 113: 449–453; 1993.
Mousses S., McAuley, L. et al. Molecular and immunohistochemical identification of p53 alterations in bone and soft tissue sarcomas. Mod. Pathol. 9: 1–6; 1996.
Nielsen-Preiss S.M., Quigley, J.P. Detection and characterization of low abundance cellular proteins that specifically increase upon loss of the metastatic phenotype. J. Cell Biochem. 51: 219–235; 1993.
Nielsen-Preiss S.M.; Quigley, J.P. et al. Co-inoculation of human and murine carcinoma cells induces reciprocal suppression of metastasis by both cell lines. Clin. Exp. Mets. 17: 489–496; 1999.
Ossowski L.; Reich, E. Loss of malignancy during serial passage of human carcinoma in culture and discordance between malignancy and transformation parameters. Cancer Res. 40: 2310–2315; 1980.
Rochet N.; Dobousset, J. et al. Establishment, characterization and partial cytokine expression profile of a new human osteosarcoma cell line (CAL 72). Int. J. Cancer 82: 282–285; 1999.
Sandberg A. and Bridge, J. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genet. Cytogenet. 145: 1–30; 2003.
Yamaguchi T.; Toguchida, J. et al. Allelotype analysis in osteosarcomas: frequent allele loss on 3q, 13q, 17p and 18q. Cancer Res. 52: 2419–2423; 1992.
Acknowledgments
We wish to thank Dr. Marileila Garcia (University of Colorado HSC) for her assistance with the karyotype analysis, Karen Helm (University of Colorado HSC) for her assistance with the flow cytometry, and Kate McInnerney and Dr. Jean Starkey (Montana State University) for their assistance and use of the equipment in the Genomics Core Facility. This work was supported in part by NIH R03 AR052898-01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
The work presented in this manuscript was initiated by the three authors at the University of Colorado Health Sciences Center, Denver, CO.
Rights and permissions
About this article
Cite this article
Gillette, J.M., Gibbs, C.P. & Nielsen-Preiss, S.M. Establishment and characterization of OS 99-1, a cell line derived from a highly aggressive primary human osteosarcoma. In Vitro Cell.Dev.Biol.-Animal 44, 87–95 (2008). https://doi.org/10.1007/s11626-007-9075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-007-9075-8