Abstract
Background
Lower urinary tract symptoms (LUTS), often secondary to benign prostatic hyperplasia, are a common problem for older men. Lifestyle factors, including physical activity and sedentariness, may be important LUTS risk factors and suitable targets for intervention.
Objective
To determine whether physical activity and sedentariness are associated with LUTS incidence and progression.
Design
The Health Professionals Follow-up Study is a prospective cohort of men that began in 1986. Follow-up for LUTS is complete through 2008.
Participants
Men aged 40–75 years at enrollment and members of health professions.
Main Measures
Total weekly metabolic equivalent of task (MET)-hour scores were calculated and were categorized (< 9, 9 to < 21, 21 to < 42, 42 to < 63, ≥ 63 MET-hours/week). Participants reported their average time/week spent sitting watching television as a measure of sedentariness, which was categorized (< 1, 1–3, 4–10, 11–29, ≥ 30 h/week). Participants completed the International Prostate Symptom Score survey and reported treatments for LUTS periodically. Cox proportional hazards regression was used to estimate multivariable-adjusted hazard ratios (HR) of physical activity and television watching with LUTS incidence and progression.
Key Results
After multivariable adjustment, including for body mass index (BMI), men with the highest physical activity were 19% (HR = 0.81, 95% CI = 0.74–0.89; p trend < 0.0001) less likely to develop incident moderate or worse LUTS than men in the lowest category. Men who watched television ≥ 30 h/week were 24% (HR = 1.24, 95% CI = 1.05–1.45; p trend = 0.004) more likely to develop incident moderate or worse LUTS than men who watched < 1 h/week. These associations persisted after mutual adjustment. We observed no associations with LUTS progression.
Conclusions
In this large prospective study, more activity and less sedentariness were associated with lower risk of incident LUTS independent of one another and BMI. Physical inactivity and sedentariness were not associated with LUTS worsening. Increasing physical activity and reducing sedentariness may be strategies for preventing LUTS in addition to their well-established benefits for other diseases.
Similar content being viewed by others
INTRODUCTION
Lower urinary tract symptoms (LUTS), often secondary to benign prostatic hyperplasia (BPH), are an important health problem for older men. About 50–70% of men over age 50 may suffer from LUTS.1 Although effective LUTS medications and surgeries exist, treatments are expensive and, given the large number of men affected, contribute to growing healthcare costs.2, 3 Lifestyle factors, including physical activity and sedentary behavior, may be LUTS risk factors1 and could be intervention targets.
Although a few randomized trials have evaluated physical activity as a LUTS intervention, a Cochrane systematic review of six studies on this topic rated quality of existing evidence as very low, concluding that additional high-quality research is necessary.4 Some observational evidence suggests that physical activity may prevent LUTS, but most studies were not prospective with incident cases. A 2008 meta-analysis of 11 studies concluded that physical activity was associated with decreased LUTS risk,5 but only 2 studies evaluated physical activity measured in men without LUTS in association with subsequent risk of incident LUTS. One of the largest included studies, conducted in the Health Professionals Follow-up Study (HPFS) cohort and published in 1998, investigated physical activity in relation to prevalent BPH/LUTS.6 Since publication of the 2008 meta-analysis, several additional studies have been published,7,8,9,10,11,12,13,14,15,16 but only three were prospective.13,14,15 All of these found that physical activity is inversely associated with LUTS incidence, although one only investigated nocturia.13 Only one study has examined physical activity and the progression of existing LUTS to a worsened state, finding no association.17 Further, only one examined sedentary behavior in relation to LUTS incidence, finding that prolonged sitting time was associated with increased risk.15
We undertook a prospective analysis examining both physical activity and sedentary behavior in relation to LUTS incidence and progression in a large cohort of middle-aged and older US men.
METHODS
Study Design and Population
This prospective analysis was conducted in the HPFS. This cohort of 51,529 US men began in 1986, enrolling men aged 40–75 years (Supplemental Methods). This study was approved by Institutional Review Boards at the Harvard TC Chan School of Public Health and Johns Hopkins Bloomberg School of Public Health. All participants provided written informed consent.
Assessment of Physical Activity and Sedentary Behavior
Beginning in 1990, and every 2 years thereafter, participants were asked to report average time per week (in 13 response categories ranging from “none” to “40+ hours/week”) spent participating in a list of physical activities (Supplemental Methods). Participants also reported usual walking speed (brisk, average, or easy) and the number of flights of stairs climbed per day. For each activity, weekly energy expenditure was calculated by multiplying usual intensity for each exercise in metabolic equivalent of task (MET)18 by median of the category of hours per week reported. Total weekly MET-hour scores were then calculated by summing these scores across all activities. Total weekly scores were categorized into 5 categories (< 9, 9 to < 21, 21 to < 42, 42 to < 63, ≥ 63 MET-hours/week), which were chosen in multiples of 3 MET-hours/week (equivalent to 1 h/week of walking at average pace), as described previously.19
The primary sedentary behavior exposure was time spent sitting watching television. This has been shown to be the surrogate of sedentariness most strongly associated with adverse health outcomes previously in HPFS.20, 21 On every questionnaire beginning in 1990, participants reported average time spent sitting watching television each week (13 response categories ranging from “none” to “40+ hours/week”). Response categories were collapsed into 5 (< 1, 1–3, 4–10, 11–29, ≥ 30 h/week) for analysis.
For joint categories of physical activity and sedentary behavior, we collapsed sedentary behavior into categories of < 30 and ≥ 30 h/week as we observed in our main analyses that this appeared to be a meaningful cutpoint. Statistical interaction was evaluated using the likelihood ratio test.
Assessment of LUTS Incidence and Progression
Details of the assessment of LUTS in the HPFS are provided in Supplemental Methods. Briefly, every 2 years since 1988, HPFS participants were asked about health conditions and medication use, including surgery for prostatic enlargement and use of finasteride and alpha-blockers for BPH.22 The International Prostate Symptom Score (IPSS)23 was assessed in 1992, 1994, 1998, 2000, and 2008.24 In addition to total IPSS, we examined irritative (frequency, urgency, and nocturia) and obstructive (incomplete emptying, intermittency, weak stream, and hesitancy) symptoms separately.
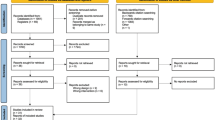
Two analytic cohorts were defined for incidence and progression. Men diagnosed with any cancer at baseline (n = 4516) were excluded from both cohorts. The incidence cohort included 25,725 men who returned the 1992 survey, did not have a cancer diagnosis in 1992 or earlier, returned a valid food frequency questionnaire in 1986, had not had surgery to treat LUTS in 1992 or earlier, had an IPSS of 0–7 in 1992, and were not missing physical activity or television watching information. We defined LUTS incidence into two ways: (1) modest or worse LUTS, i.e., “modest” defined as IPSS of ≥ 8 or surgery or medication use (n = 10,845) and (2) moderate or worse LUTS, i.e., “moderate” defined as IPSS of ≥ 15 or surgery or medication use (n = 5713). Surgery or medication use was included as a “case” for both definitions of LUTS incidence regardless of reported IPSS because these interventions likely improve the score making it an unreliable measure of underlying condition; we assumed that LUTS requiring treatment were severe. Men were censored at the time of diagnosis if they developed prostate cancer during follow-up (n = 1639).
For progression, men entered the analytic cohort when they first reported an IPSS of 8 to 14 but did not have cancer then or earlier, returned a valid FFQ in 1986, had not had surgery or used medications for LUTS, and were not missing physical activity or television watching information. The progression cohort included 9019 men. We defined LUTS progression into two ways: (1) moderate or worse LUTS, i.e., “moderate”, defined as IPSS of ≥ 15 or surgery or medication use (n = 3162) and (2) severe LUTS, i.e., “severe” defined as IPSS of ≥ 20 or surgery or medication use (n = 2325). LUTS requiring either medication or surgery were assumed to be severe and, therefore, use of these treatments was a qualifying criterion for both definitions of LUTS progression. For both LUTS incidence and progression, we focused on the results for the more stringent definition because the less stringent definition may reflect a broader constellation of underlying pathologies or contributors to LUTS. As previously reported, the age-standardized incidence and progression rates in HPFS are as follows: per 1000 person-years, LUTS incidence moderate = 18.5, modest = 40.5, LUTS progression to severe = 44.9 to moderate = 63.0.22
Statistical Analysis
We used Cox proportional hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) of LUTS incidence and progression. Physical activity and television watching were modeled as time-dependent variables (simple updating). All models were adjusted for age (years). Multivariable models were further adjusted for factors known or hypothesized to be associated with physical activity or sedentary behavior and LUTS: body mass index (BMI); smoking history; dietary intake of total energy, polyunsaturated fatty acids, fruit, vegetables, red meat, and alcohol; use of supplemental vitamin E and selenium; and aspirin use. For physical activity, linear trend across categories was assessed by entering into the model continuous weekly MET-hour score variable and assessing its significance using the Wald test. For television watching, the linear trend was assessed by creating a variable assigning to each category median hours per week for that category and entering that variable into the model and assessing its significance using the Wald test.
We conducted sensitivity analyses examining baseline physical activity and television watching to assess whether past exposure might be differently associated with LUTS incidence or progression than recent exposure. We conducted sensitivity analyses updating exposures as a cumulative average to minimize random within-person variation and to better represent an individual’s long-term average physical activity or television watching.
We conducted analyses of both physical activity and television watching stratified by BMI (< 25 versus ≥ 25 kg/m2). Statistical interaction was evaluated using the likelihood ratio test.
RESULTS
Age-standardized characteristics of the analytic cohort by categories of baseline total physical activity are shown in Table 1. Men who were more physically active had lower BMI; were less likely to have smoked in the 10 years prior to baseline; consumed more total energy, dietary beta-carotene, lutein, fruits, vegetables, and cruciferous vegetables; were more likely to use vitamin E and selenium supplements; and were less likely to report high blood pressure or diabetes (Table 1). In the incidence cohort, 91.8% of participants were White and 0.7% were African American; in the progression cohort, 91.7% were White and 0.8% were African American.
LUTS Incidence
After adjustment for age, men with the highest total physical activity were statistically significantly less likely to develop incident modest (IPSS ≥ 8) or moderate (IPSS ≥ 15) LUTS compared with men in the lowest category (≥ 63 versus < 9 MET-hour/week: modest HR = 0.90, 95% CI = 0.84–0.95, p trend < 0.0001; moderate HR = 0.80, 95% CI = 0.74–0.87, p trend < 0.0001; Table 2). Results were nearly identical after multivariable adjustment, including for BMI (≥ 63 versus < 9 MET-hour/week: modest HR = 0.90, 95% CI = 0.84–0.96, p trend < 0.0001; moderate HR = 0.81, 95% CI = 0.74–0.89, p trend < 0.0001; Table 2; further adjusted for waist, moderate HR = 0.82, 95% CI = 0.75–0.90, p trend = 0.0002). Results were similar when baseline or cumulative updated physical activity was used (≥ 63 versus < 9 MET-hour/week: at baseline—modest HR = 0.86, 95 CI = 0.80–0.92, p trend < 0.0001 and moderate HR = 0.82, 95% CI = 0.75–0.90, p trend = 0.006; cumulative updated—modest HR = 0.80, 95% CI = 0.74–0.87, p trend < 0.0001 and moderate HR = 0.77, 95% CI = 0.69–0.86, p trend < 0.0001).
After adjustment for age, men who watched the most television had a statistically significantly increased risk of modest (IPSS ≥ 8) and moderate (IPSS ≥ 15) LUTS compared with men who watched the least television (≥ 30 versus < 1 h/week: modest HR = 1.32, 95% CI = 1.17–1.50, p trend < 0.0001 and moderate HR = 1.27, 95% CI = 1.08–1.49, p trend = 0.001; Table 2). Results were nearly identical after multivariable adjustment (≥ 30 versus < 1 h/week: modest HR = 1.28, 95% CI = 1.13–1.45, p trend < 0.0001 and moderate HR = 1.24, 95% CI = 1.05–1.45, p trend = 0.004; Table 2; further adjusted for waist, moderate HR = 1.23, 95% CI = 1.05–1.44, p trend = 0.006). Results were consistent when baseline or cumulative updated sedentary time was used (≥ 30 versus < 1 h/week: baseline—modest HR = 1.21, 95% CI = 1.04–1.41, p trend < 0.0001 and moderate HR = 1.13, 95% CI = 0.93–1.37, p trend = 0.06; cumulative updated—modest HR = 1.29, 95% CI = 1.05–1.59, p trend < 0.0001 and moderate HR = 1.21, 95% CI = 0.88–1.67, p trend = 0.0004).
Associations of physical activity and television watching with irritative and obstructive LUTS were similar to LUTS overall (Supplementary Table 1). Findings were unchanged when men with diabetes (≥ 63 versus < 9 MET-hour/week: moderate HR = 0.80, 95% CI = 0.73–0.88, p trend < 0.0001; ≥ 30 versus < 1 h television/week: moderate HR = 1.25, 95% CI = 1.06–1.48, p trend = 0.002), diuretics users (≥ 63 versus < 9 MET-hour/week: moderate HR = 0.82, 95% CI = 0.75–0.91, p trend = 0.0006; ≥ 30 versus < 1 h television/week: moderate HR = 1.29, 95% CI = 1.07–1.54, p trend = 0.005), or anti-depressant medications users (≥ 63 versus < 9 MET-hour/week: moderate HR = 0.82, 95% CI = 0.75–0.90, p trend = 0.0001; ≥ 30 versus < 1 h television/week: moderate HR = 1.24, 95% CI = 1.05–1.46, p trend = 0.005) were excluded.
Associations for physical activity (inverse) and television watching (positive) with LUTS were independent of each other as mutual adjustment did not alter these findings (Table 2). When we examined joint categories of physical activity and television watching, we observed that, particularly for moderate LUTS, compared to those who were least active and watched the most television, those who were most active and watched the least television had the lowest LUTS risk (Fig. 1). Within each category of television watching, there was a strong inverse association with increasing activity; we also observed a modest association with increased television watching within categories of activity (Fig. 1). There was no evidence of statistical interaction between physical activity and sedentary behavior (p interaction: modest = 0.39, moderate = 0.76).]-->
Examining vigorous physical activity separately, it was inversely associated with incidence of both modest (IPSS ≥ 8) and moderate (IPSS ≥ 15) LUTS, but the association was not as strong as for total physical activity (Supplementary Table 2). We also examined whether walking was associated with LUTS and found that there was a modest trend toward decreasing risk of incident LUTS, particularly for moderate (IPSS ≥ 15) LUTS, with increased walking (Supplementary Table 2). For incidence of moderate LUTS, for which we saw the clearest evidence for a benefit of physical activity, we examined brisk walking, jogging, and biking separately and did not observe any statistically significant associations (brisk waking vs. none: > 0 to < 1.5 h/week, HR = 1.04, 95% CI = 0.95–1.13; ≥ 1.5 h/week, HR = 0.96, 95% CI = 0.90–1.02).
We observed no differences in the association between physical activity and incident LUTS between those were and were not obese (Supplementary Table 3). We observed no statistical interaction with age.
LUTS Progression
We observed no association between either physical activity or television watching and progression to moderate (IPSS ≥ 15) or severe (IPSS ≥ 20) LUTS (Table 3). Findings were unchanged when men with diabetes (≥ 63 versus < 9 MET-hour/week: severe HR = 0.93, 95% CI = 0.80–1.08, p trend = 0.85; ≥ 30 versus < 1 h television watching/week: severe HR = 1.15, 95% CI = 0.88–1.50, p trend = 0.61) or diuretic medication users (≥ 63 versus < 9 MET-hour/week: severe HR = 0.92, 95% CI = 0.78–1.07, p trend = 0.94; ≥ 30 versus < 1 h television watching/week: severe HR = 1.20, 95% CI = 0.90–1.60, p trend = 0.99) were excluded. When combined effects of physical activity and television watching were considered, no pattern of association was observed for LUTS progression (p interaction: moderate = 0.24, severe = 0.48).
DISCUSSION
In this large prospective study, we observed that more total physical activity and less television watching were independently associated with lower LUTS risk. Vigorous physical activity was less strongly associated with LUTS than total activity, whereas a lower intensity physical activity, walking, was associated with lower LUTS risk, likely due to lower participation in vigorous versus lower intensity activity in this cohort. Our findings suggest that higher total MET-hours/week of activity, which can be achieved through increased duration or intensity, are associated with a lower LUTS risk. We observed increasing benefit across categories of increasing total physical activity with greatest benefit observed for men doing ≥ 63 MET-hours/week of activity, equivalent to walking 3+ hours/day at a moderate pace. Current physical activity guidelines recommend that adults do 150 min/week of moderate activity or 75 min/week of vigorous activity,25 equating to approximately 10 MET-hours/week and falling within our second category of exposure. This suggests that meeting these guidelines will provide modest protection against moderate LUTS, but no protection for modest LUTS; exceeding these guidelines provided the most benefit.
In contrast to LUTS risk, we observed no association between physical activity or sedentary behavior and LUTS progression. Our results for both LUTS incidence and progression were very similar when we used baseline and cumulative updated measures, rather than simple updated. This is likely because activity patterns among the men in our study generally did not change markedly over time, making each of these approaches to defining exposure similar to one another.
Our findings are consistent with previous prospective studies that have found an inverse association between physical activity and LUTS risk.5, 12,13,14,15 In our previous HPFS study, total physical activity was inversely associated with prevalent LUTS, with walking, their most common activity, also being inversely associated with LUTS.6 Our current findings for incident LUTS are very similar to our prior findings for prevalent LUTS. In addition, our null finding for physical activity and LUTS progression is consistent with the one previous study on this topic.17 In the HPFS study of prevalent LUTS, we reported a positive association between sedentary time and LUTS,6 which we now observed in the present analysis for incident LUTS. Our results are also consistent with those from a recently published study in a Korean population that found that physical activity (inverse) and sedentary time (positive) were both related to LUTS incidence independently.15
Several biologic mechanisms exist by which physical activity and sedentary behavior may influence LUTS risk. One mechanism is their influence on weight gain, although we found physical activity and television watching were associated with LUTS risk independent of BMI, and in both overweight/obese and normal weight men. However, BMI may not adequately control for visceral adiposity. Physical activity can result in meaningful reductions in visceral fat with accompanying improvements in metabolic status, without dramatically changing overall BMI.26 Thus, we cannot rule out an influence of physical activity and sedentary behavior on weight gain as the mechanism by which they may be influencing LUTS. Another mechanism is their effects on testosterone levels,27 which may in turn affect prostate tissue hyperplasia.28 Endothelial and vascular dysfunction may lead to LUTS, and dysfunctions in these pathways can be improved by increasing physical activity and decreasing sedentariness.27 Finally, physical activity and sedentary behavior influence systemic inflammation, which is also related to LUTS.27, 29 Studies of the biologic mechanisms of physical activity and sedentary behavior have demonstrated that, while they influence similar biologic pathways, their activities are not merely complementary and their biologic actions differ from one another.27 Our results support this paradigm and suggest that either increasing physical activity or reducing sedentary time may have beneficial effects on LUTS risk, and that doing both may have the largest impact.
Strengths of our study include the prospective design, measures of physical activity and television watching every 2 years, very large sample size, and control for multiple potential confounding factors. One possible limitation is that our measures of physical activity and television watching are self-reported. However, due to our prospective design, any misclassification due to self-report is most likely to be non-differential, which would tend to bias findings toward the null. Thus, the true associations may be stronger than those we report here. Another possible limitation is that we studied subjective measures of pathologies underlying LUTS. However, LUTS are what men experience and, thus, should be the basis for the development of preventive lifestyle strategies. In addition, findings persisted after excluding men with diabetes or who were using diuretic medications, which can cause symptoms similar to LUTS.
CONCLUSIONS
In this large, prospective study, more total physical activity and less television watching were associated with a lower LUTS risk independent of one another and BMI. Physical activity and sedentary behavior were not associated with LUTS progression. Increasing physical activity and reducing sedentariness may be strategies for LUTS primary prevention in addition to their well-established benefits for other diseases.
References
Egan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clin North Am 2016;43(3):289–97.
Kirby RS, Kirby M, Fitzpatrick JM. Benign prostatic hyperplasia: counting the cost of its management. BJU Int 2010;105(7):901–2.
Urologic Diseases in America. In: Litwin MS, Saigal CS, eds. Washington, DC: US Government Printing Office: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2012.
Silva V, Grande AJ, Peccin MS. Physical activity for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database Syst Rev 2019;4:CD012044.
Parsons JK, Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur Urol 2008;53(6):1228–35.
Platz EA, Kawachi I, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, et al. Physical activity and benign prostatic hyperplasia. Arch Intern Med 1998;158(21):2349–56.
Fowke JH, Phillips S, Koyama T, Byerly S, Concepcion R, Motley SS, et al. Association between physical activity, lower urinary tract symptoms (LUTS) and prostate volume. BJU Int 2013;111(1):122–8.
Lagiou A, Samoli E, Georgila C, Minaki P, Barbouni A, Tzonou A, et al. Occupational physical activity in relation with prostate cancer and benign prostatic hyperplasia. Eur J Cancer Prev 2008;17(4):336–9.
Litman HJ, Steers WD, Wei JT, Kupelian V, Link CL, McKinlay JB. Relationship of lifestyle and clinical factors to lower urinary tract symptoms: results from Boston Area Community Health survey. Urology. 2007;70(5):916–21.
Maso LD, Zucchetto A, Tavani A, Montella M, Ramazzotti V, Polesel J, et al. Lifetime occupational and recreational physical activity and risk of benign prostatic hyperplasia. Int J Cancer 2006;118(10):2632–5.
Orsini N, RashidKhani B, Andersson S-O, Karlberg L, Johansson J-E, Wolk A. Long-term physical activity and lower urinary tract symptoms in men. J Urol 2006;176(6):2546–50.
Williams PT. Effects of running distance and performance on incident benign prostatic hyperplasia. Med Sci Sports Exerc 2008;40(10):1733.
Wolin KY, Grubb III RL, Pakpahan R, Ragard L, Mabie J, Andriole GL, et al. Physical activity and benign prostatic hyperplasia-related outcomes and nocturia. Med Sci Sports Exerc 2015;47(3):581.
Penson DF, Munro HM, Signorello LB, Blot WJ, Fowke JH. Obesity, physical activity and lower urinary tract symptoms: results from the Southern Community Cohort Study. J Urol 2011;186(6):2316–22.
Park HJ, Park CH, Chang Y, Ryu S. Sitting time, physical activity and the risk of lower urinary tract symptoms: a cohort study. BJU Int 2018;122(2):293–9.
De Nunzio C, Nacchia A, Cicione A, Cindolo L, Gacci M, Cancrini F, et al. Physical Activity as a Protective Factor for Lower Urinary Tract Symptoms in Male Patients: A Prospective Cohort Analysis. Urology. 2019;125:163–8.
Rohrmann S, Katzke VA, Kaaks R. Lifestyle and progression of lower urinary tract symptoms in German men-results from the EPIC-Heidelberg cohort. Urology. 2018;120:192–196.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25(1):71–80.
Keum N, Bao Y, Smith-Warner SA, Orav J, Wu K, Fuchs CS, et al. Association of Physical Activity by Type and Intensity With Digestive System Cancer Risk. JAMA Oncol 2016;2(9):1146–53.
Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–91.
Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. 2014;106(7). https://doi.org/10.1093/jnci/dju098
Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J Urol 2012;188(2):496–501.
Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 1992;148:1549–57.
Rohrmann S, Giovannucci E, Willett WC, Platz EA. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr 2007;85:523–9.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8.
Giovannucci E. An Integrative Approach for Deciphering the Causal Associations of Physical Activity and Cancer Risk: The Role of Adiposity. J Natl Cancer Inst 2018;110(9):935–41.
Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012;2(2):1143–211.
Giovannucci E, Rimm EB, Chute CG, Kawachi I, Colditz GA, Stampfer MJ, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol 1994;140(11):989–1002.
Ficarra V, Rossanese M, Zazzara M, Giannarini G, Abbinante M, Bartoletti R, et al. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep 2014;15(12):463.
Funding
This study was financially supported by Urological Diseases in America Project N01 DK70003, and Department of Health and Human Services, National Institutes of Health Public Health Service Grants R01 DK45779, P01 CA55075, P50 DK082998, and UM1CA167552.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by Institutional Review Boards at the Harvard TC Chan School of Public Health and Johns Hopkins Bloomberg School of Public Health. All participants provided written informed consent.
Conflict of Interest
Dr. Platz received personal fees from the American Association for Cancer Research for her role as Editor-in-Chief and from Kaiser-Permanente for her role as an external advisor to their cancer research program. Drs. Mondul and Giovannucci have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mondul, A.M., Giovannucci, E. & Platz, E.A. A Prospective Study of Physical Activity, Sedentary Behavior, and Incidence and Progression of Lower Urinary Tract Symptoms. J GEN INTERN MED 35, 2281–2288 (2020). https://doi.org/10.1007/s11606-020-05814-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-020-05814-1