Abstract
Background
Twenty-eight states have passed breast density notification laws, which require physicians to inform women of a finding of dense breasts on mammography.
Objective
To evaluate changes in breast cancer stage at diagnosis after enactment of breast density notification legislation.
Design
Using a difference-in-differences analysis, we examined changes in stage at diagnosis among women with breast cancer in Connecticut, the first state to enact legislation, compared to changes among women in control states. We used data from the Surveillance, Epidemiology, and End Results Program (SEER) registry, 2005–2013.
Participants
Women ages 40–74 with breast cancer.
Intervention
Breast density notification legislation, enacted in Connecticut in October of 2009.
Main Measure
Breast cancer stage at diagnosis.
Key Results
Our study included 466,930 women, 25,592 of whom lived in Connecticut. Legislation was associated with a 1.38-percentage-point (95 % CI 0.12 to 2.63) increase in the proportion of women in Connecticut versus control states who had localized invasive cancer at the time of diagnosis, and a 1.12-percentage-point (95 % CI −2.21 to −0.08) decline in the proportion of women with ductal carcinoma in situ at diagnosis. Breast density notification legislation was not associated with a change in the proportion of women in Connecticut versus control states with regional-stage (−0.09 percentage points, 95 % CI −1.01 to 1.02) or metastatic disease (−0.24, 95 % CI −0.75 to 0.28). County-level analyses and analyses limited to women younger than 50 found no statistically significant associations.
Limitations
Single intervention state, limited follow-up, potential confounding from unobserved trends.
Conclusions
Breast density notification legislation in Connecticut was associated with a small increase in the proportion of women diagnosed with localized invasive breast cancer in individual-level but not county-level analyses. Whether this finding reflects potentially beneficial early detection or potentially harmful overdiagnosis is not known. Legislation was not associated with changes in regional or metastatic disease.
Similar content being viewed by others
INTRODUCTION
Among women undergoing routine screening mammography, about 43 % have radiographically dense breasts.1 Dense breasts are considered normal, but the finding has two important implications. First, breast density may be an independent, moderate risk factor for breast cancer.2 Second, the sensitivity and specificity of mammography are reduced among women with dense breasts, although newer digital techniques partially mitigate this effect.3
Given the limitations of mammography, some have advocated supplemental screening with magnetic resonance imaging (MRI) or ultrasound in women with dense breasts. Indeed, each of these modalities can detect additional cases of breast cancer after a negative mammogram in women with dense breasts. Ultrasound, for example, detects about 5 cancers per 1000 women screened, while MRI can detect between 3 and 33 cases of cancer.4 Supplemental screening most commonly detects localized invasive cancers rather than ductal carcinoma in situ (DCIS) or late-stage disease.5
Supplemental screening tests, however, have poor positive predictive value, prompting concerns that widespread use among low-risk women could generate a considerable number of false positives and subsequent invasive testing.5 – 9 Furthermore, there are no randomized trials or observational studies evaluating the effect of adjunctive screening on morbidity or mortality.4 , 10 The US Preventive Services Task Force 2016 guideline on screening for breast cancer determined that there was insufficient evidence to recommend for or against supplemental screening.11
Despite uncertainty about the optimal approach to screening women with dense breasts, 28 states have passed breast density notification laws. These laws vary by state, but generally require that physicians disclose a finding of dense breasts to women undergoing mammography.12 A number of states also require that physicians inform women with dense breasts that they may benefit from adjunctive screening with ultrasound or MRI, and some states (including Connecticut) require that private insurers cover supplemental screening for women with dense breasts. Although the number of states with breast density notification legislation has grown steadily, no study has examined the effect of this kind of legislation on health outcomes for women.
Our goal was to evaluate the relationship between breast density notification legislation and stage at diagnosis among women with breast cancer. Breast density notification legislation may lead to an increased use of supplemental screening, and supplemental screening, in turn, might lead to an increase in early-stage breast cancer diagnosis. Thus, we evaluated changes in cancer stage at the time of diagnosis among women living in Connecticut, the first state subject to breast density notification, compared to control populations.
As a secondary goal, we used a county-level analysis to examine changes in stage-specific incidence associated with breast density notification legislation. If legislation enabled early detection of cancers that would have eventually progressed to late-stage cancer, legislation should be associated with increased incidence of early-stage cancer and decreased incidence of late-stage cancer. In contrast, if legislation facilitated detection of DCIS or early invasive cancers that did not progress and become harmful (i.e., overdiagnosis), legislation would be associated with increased DCIS or early-stage invasive cancer without a concomitant drop in late-stage cancer.
METHODS
Study Design
We used two complementary approaches to examine the association between breast density notification legislation and changes in breast cancer stage at diagnosis. In our primary approach, we evaluated the proportion of women with DCIS, localized, regional, or metastatic disease at the time of diagnosis. We used a difference-in-differences design to compare changes in the proportion of women diagnosed at each stage in Connecticut, the first state subject to breast density notification legislation, to changes among women in states without such legislation.
In a second, complementary difference-in-differences analysis, we evaluated changes in stage-specific incidence in counties in Connecticut and control counties. The goal of this analysis was to describe changes in incidence associated with breast density notification legislation and to evaluate for evidence of overdiagnosis associated with this policy. Overdiagnosis refers to detecting a cancer, usually by screening, that would never have been clinically apparent. In the case of breast cancer, screening contributes to overdiagnosis of both in situ and early-stage invasive cancers.13
Stage-specific incidence patterns over time can provide evidence of an effective or ineffective screening program. As noted, an effective screening program should result in an increase in the incidence of early-stage disease as early-stage cancers are detected, and a decline in late-stage disease as advanced disease is averted by early diagnosis.14 In contrast, an increased incidence of early-stage disease without a concomitant decline in late-stage disease suggests that the screening program detects only clinically insignificant cancers and contributes to overdiagnosis.15 Using this framework, we evaluated changes in stage-specific incidence in counties in Connecticut and control counties.
Data Sources, Variables, and Participants
We analyzed incident breast cancer cases from the Surveillance, Epidemiology, and End Results Program (SEER) Registry.16 SEER is a population-based registry that captures cancer incidence and mortality in specific geographies. SEER covers 13 US states (Alaska, California, Connecticut, Georgia, Hawaii, Kentucky, Iowa, Louisiana, Michigan, New Jersey, New Mexico, Utah, Washington). Among these, two states, Connecticut and California, passed breast density notification legislation during the period for which data were available. Connecticut enacted its legislation in October of 2009,17 and California in April of 2013.18 Because we had less than 1 year of follow-up data for California, and because we would not expect to see a substantial effect from legislation in this short time, we excluded data from the post-legislation period in California in our main analyses. In a sensitivity analysis, we included all California data.
In our main analyses, we included women residing in all SEER areas who were between the ages of 40 and 74 and who had a diagnosis of invasive breast cancer or DCIS. We excluded women with a diagnosis of lobular carcinoma in situ (LCIS), as LCIS is typically not detected during screening.19 We also excluded women who had unstaged cancer. We excluded data from counties affected by Hurricane Katrina in 2005, which are not considered part of the standard SEER data set. We used US Census county populations to calculate incidence. The Area Resource File for 2013–2014 and 2014–2015 provided county-level covariates.20 We limited our analysis to the period from 2005 to 2013.
Our main outcome variable was stage at diagnosis. We used a summary staging variable prepared by SEER which collapses stage at diagnosis into four basic categories: in situ, localized, regional, and distant/metastatic.21 Localized disease includes cancer confined to the breast, and regional disease includes local extension outside the confines of the breast and to regional lymph nodes. In a sensitivity analysis, we used an alternate summary staging variable which uses a more stringent definition of metastatic disease.
Statistical Analysis
For our individual-level analysis, we used a multinomial logistic regression model with stage at diagnosis as the outcome. This model included an indicator variable for women residing in Connecticut, an indicator for time period pre- and post-legislation (01/2005–09/2009, 10/2009–12/2013), and the interaction between the two, which captures effects associated with breast density notification legislation. We also adjusted for race, ethnicity, age, and the post-legislation time period in California. We expressed results as predicted probabilities. We assessed for pre-legislation trends in our main analysis by interacting time (year) with treatment group during the pre-legislation period.
For our county-level analysis, we fit a series of mixed-effects negative binomial models of change in stage-specific incidence over time. As in our individual-level models, we included a binary term to represent counties in Connecticut, a binary term for the pre/post-legislation time period, and the interaction. Here, we defined the pre- and post-legislation periods as 2005–2009 and 2010–2013, respectively. Models included an exposure term to account for county population (total population of women in the age range of interest in the county) and county-specific random effects to account for repeated measures on each county. We incorporated time-varying covariates derived from the Area Resource File including median household income, percentage of the female population that is white, percentage uninsured, percentage of women aged 40–64, and physicians per capita.
Subgroup Analyses
For both analytic approaches, we evaluated stage at diagnosis among women ages 40–49, given that dense breasts are more common among younger women.1 We also evaluated women ages 50–74, because while dense breasts are less common, screening overall is more common in this population.22
Sensitivity Analyses
Connecticut enacted breast density notification legislation in late 2009. In a sensitivity analysis, we excluded the first year post-legislation in our individual-level model, as it was a transitional period. We also estimated a model that included post-legislation data from California in 2013.
Power Calculation
For our county-level analyses, we used a simulation-based method to estimate the minimum effect size detectible with ≥80 % power for our effect of interest, the change in incidence in counties in Connecticut compared to control counties, in our county-level model.
RESULTS
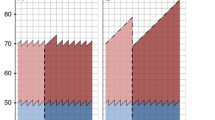
Our individual-level analysis included 504,376 women ages 40–74 who were diagnosed with breast cancer between 2005 and 2013. We excluded 5515 women who did not have staging data available, 13,616 women with LCIS, and 18,315 who were diagnosed in California between April and December 2013. Our final analysis included 466,930 women with a breast cancer diagnosis. Of these, 25,592 lived in Connecticut at the time of diagnosis. Characteristics of the women in our sample are reported in Table 1. Women in Connecticut were more likely to be white, and a larger proportion had DCIS at diagnosis than did women in other states. Figure 1 depicts the proportion of women diagnosed at each stage in Connecticut and in control states by year.
Stage at diagnosis among women in Connecticut and control states. Panel A depicts the distribution of stage at diagnosis among women ages 40–74 with breast cancer in control states. Panel B depicts the distribution of stage at diagnosis among women ages 40–74 with breast cancer in Connecticut. *Breast density notification legislation was enacted in October of 2009.
Our individual-level analysis examined changes in the proportion of women diagnosed at each stage over time among women in Connecticut and control states. We found that the proportion of women with a localized cancer increased from 50.0 % to 52.4 % for women in Connecticut during the post-legislation period. We observed a smaller increase in control states, from 50.6 % to 51.6 %. Overall, we found a 1.38-percentage-point (95 % CI 0.12 to 2.63) increase in the proportion of women diagnosed with localized breast cancer in Connecticut post-legislation, after accounting for trends in non-legislation states. We also found a 1.12-percentage-point decrease (95 % CI −2.21 to −0.08) post-legislation in the proportion of women diagnosed with DCIS in Connecticut, after accounting for changes in control states. Changes in the proportion of women diagnosed with regional-stage disease (−0.09 percentage points, 95 % CI −1.01 to 1.02) and metastatic disease (−0.24 percentage points, 95 % CI −0.75 to 0.28) did not differ between Connecticut and control states (Table 2). Among women ages 40–49 and women ages 50–74, we did not observe statistically significant changes in the proportion of women diagnosed at each stage in Connecticut compared to control states, though effect sizes were similar to what we observed in our main analysis (Table 2).
When assessing pre-legislation trends, we found that the proportion of women with metastatic disease at the time of diagnosis declined slightly in Connecticut while remaining stable in control states (approximate 0.30 percentage-point decline per year, p = 0.02). In our main analysis, however, we did not observe a decline in the proportion of women diagnosed with metastatic disease in Connecticut compared to control states, even though these divergent trends could have biased us toward such a finding. We did not observe divergent pre-legislation trends for the other three disease stages.
In a sensitivity analysis, we used an alternate staging system and found similar results (Online Appendix Table 1). Excluding the first post-policy year did not alter our results substantively (Online Appendix Table 2). In an analysis in which we included post-legislation data from California, we observed a small decline in the proportion of women with metastatic disease at the time of diagnosis among women in states with breast density notification legislation (−0.37 percentage points, 95 % CI −0.59 to −0.14; Online Appendix Table 3). This finding was only observed in models that included post-legislation data from California.
Our county-level analysis examined changes in stage-specific incidence and included 611 counties over a 9-year period. Eight of these counties were in Connecticut and thus were subject to breast density notification legislation from late 2009 onward. Online Appendix Table 4 describes the counties in our sample.
Figure 2 depicts age-adjusted breast cancer incidence by stage in Connecticut and in control states from 2005 to 2013. Stage-specific incidence appears relatively flat in the post-policy period in both Connecticut and control states.
In adjusted models, we found no statistically significant changes in the incidence of DCIS (incidence rate ratio [IRR] 0.96, 95 % CI 0.90 to 1.03), localized disease (IRR 1.02, 95 % CI 0.98 to 1.01), regional-stage disease (IRR 0.98, 95 % CI 0.92 to 1.04), or metastatic disease (IRR 0.98, 95 % CI 0.86 to 1.12) in counties in Connecticut compared to control counties (Table 3). We estimated that we had 80 % power to detect a 23 % change in incidence of DCIS in counties in Connecticut compared to other counties, a 13 % change in localized disease, a 16 % increase in regional-stage disease, and a 27 % change in metastatic disease at an alpha = 0.05 level.
DISCUSSION
To the best of our knowledge, ours is the first study to evaluate the effect of breast density notification legislation on breast cancer stage at diagnosis. We found that passage of legislation in Connecticut was associated with a small increase in the proportion of women diagnosed with localized invasive cancer, after accounting for trends in states without breast density notification legislation. We also observed a small decline in the proportion of women diagnosed with DCIS. Although not definitive, these findings could be explained by an effect of breast density legislation on supplemental screening, which can detect additional early-stage invasive cancers.4 In a sensitivity analysis in which we included data from California, we observed a small decline in the proportion of women with metastatic disease on presentation. This finding is driven by data from California, and is not likely attributable to passage of breast density notification legislation, as such legislation had only been in place for a matter of months in California, and would have been very unlikely to have had an effect on metastatic disease within that time frame.
Our main findings have two potential interpretations, each with important implications. First, it is possible that because of breast density notification legislation, more women in Connecticut have been diagnosed with an early-stage cancer and have been spared an eventual late-stage diagnosis. This early-stage diagnosis may translate into important health benefits, as these women may avoid the morbidity and mortality associated with a late-stage diagnosis. It is also possible, though, that at least some of this increase in early-stage diagnosis is due to overdiagnosis. In this case, the additional cancers detected are not clinically significant, and finding them does not translate into an improvement in health.
We pursued a county-level analysis to examine the association between legislation and stage-specific incidence and to help distinguish these possibilities. Specifically, given stable underlying disease rates, an increased incidence of early-stage diagnosis with an associated decrease in late-stage diagnosis would suggest that legislation promotes successful detection of early-stage disease and averts late-stage diagnoses.14 , 15 In contrast, an increase in incidence of early-stage disease, with no change in late-stage disease, would suggest that legislation may contribute to overdiagnosis. We did not detect a reduction in late-stage cancer in Connecticut, a pattern that is consistent with the explanation that supplemental screening has led to overdiagnosis. Our sample, however, had limited power to detect the small effects we saw in our individual-level analysis. Furthermore, changes in late-stage disease may take years to appear, given the natural history of breast cancer, and 4 years of follow-up may not be sufficient to observe a change. Thus, whether breast density notification legislation has led to improved health outcomes in Connecticut remains an open question.
Our findings should be interpreted with caution. First, residual confounding may explain our results. While a difference-in-differences analysis is robust to confounding from group differences that are constant over time, changes that occurred around the time of policy enactment and differentially affected Connecticut could explain our results. We have controlled for key demographics which may change differentially over time and could also affect screening rates, but residual confounding remains possible.
Second, we examined four disease stages using two separate modeling strategies, an approach which contributes to an increased likelihood of a spurious finding. With a correction for multiple testing, our findings would not be statistically significant. Still, our main findings are consistent with what we might expect to observe a priori: if breast density legislation were to have any effect, it would be to increase diagnoses of localized invasive disease. Additionally, while little is known about how breast density notification laws have affected practice, there is some indication that some radiologists in Connecticut have implemented programs to facilitate supplemental screening, and the use of supplemental screening may have increased.23 Such changes in utilization could lead to a greater proportion of women diagnosed at an early stage.
An assessment of the effect of breast density legislation with the data we used has inherent limitations. It is possible that physicians did not provide notification as mandated or that many women who received a notification took no action, and it is also possible that women in control states were notified even in the absence of legislation. A careful analysis of supplemental screening patterns in Connecticut and control states could potentially support a link between breast density notification legislation and stage at diagnosis, but such information is not available from the data source we used.
Finally, given demographic, economic, and cultural differences, findings from Connecticut may not be applicable to other states. Connecticut’s experience may also represent the case in which breast density notification legislation would be most likely to lead to a change in breast cancer detection, as the law has received considerable attention there, resulting in widespread awareness.24 In addition, Connecticut has an insurance provision that requires private insurers to cover supplemental screening for women with dense breasts, which would likely facilitate access if women did indeed wish to pursue supplemental screening. However, the majority of states with breast density notification legislation do not mandate insurance coverage for supplemental screening.
In summary, breast density notification legislation is associated with a small increase in the proportion of women diagnosed with early-stage invasive breast cancer. Given limited follow-up, we cannot determine whether this change has led to improved health outcomes or reflects overdiagnosis. A longer observation period and study of the effect of legislation in other states will help in further assessing the impact of breast density legislation.
References
Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014; 106(10). doi:10.1093/jnci/dju255.
Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. doi:10.1056/NEJMoa062790.
Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493–502. doi:10.7326/0003-4819-155-8-201110180-00005.
Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016. doi:10.7326/M15-1789.
Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–404. doi:10.1001/jama.2012.388.
Kriege M, Brekelmans CTM, Obdeijn IM, et al. Factors affecting sensitivity and specificity of screening mammography and MRI in women with an inherited risk for breast cancer. Breast Cancer Res Treat. 2006;100(1):109–19. doi:10.1007/s10549-006-9230-z.
Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers R-D, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–10. doi:10.1200/JCO.2013.52.5386.
Sprague BL, Stout NK, Schechter C, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–66. doi:10.7326/M14-0692.
Scheel JR, Lee JM, Sprague BL, Lee CI, Lehman CD. Screening ultrasound as an adjunct to mammography in women with mammographically dense breasts. Am J Obstet Gynecol. 2015;212(1):9–17. doi:10.1016/j.ajog.2014.06.048.
Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;4:CD009632. doi:10.1002/14651858.CD009632.pub2.
Siu AL. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–96. doi:10.7326/M15-2886.
Are You Dense :: Twenty-Four State Density Reporting Laws & a Growing Number of Introduced Bills in 2016. http://www.areyoudense.org/news-events/twenty-four-state-density-reporting-laws-growing-number-introduced-bills-2016/. Accessed September 27, 2016.
Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):256. doi:10.7326/M15-0970.
Chu KC, Kramer BS, Smart CR. Analysis of the role of cancer prevention and control measures in reducing cancer mortality. J Natl Cancer Inst. 1991;83(22):1636–43.
Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi:10.1056/NEJMoa1206809.
SEER Data, 1973-2012. http://seer.cancer.gov/data/. Accessed September 27, 2016.
Public Act No. 09-41 for Substitute Senate Bill No. 458. https://www.cga.ct.gov/2009/ACT/PA/2009PA-00041-R00SB-00458-PA.htm. Accessed SEptember 27, 2016.
Bill Text - SB-1538 Health care: mammograms. http://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201120120SB1538. Accessed September 27, 2016.
Oliveira TMG, Elias J, Melo AF, et al. Evolving concepts in breast lobular neoplasia and invasive lobular carcinoma, and their impact on imaging methods. Insights Imag. 2014;5(2):183–94. doi:10.1007/s13244-014-0324-6.
AHRF: Area Health Resources Files. http://ahrf.hrsa.gov/download.htm. Accessed September 27, 2016.
RECODED SEER RESEARCH DATA RECORD DESCRIPTION 2015 - http://seer.cancer.gov/data/seerstat/nov2014/TextData.FileDescription.pdf. Accessed September 27, 2016.
Block LD, Jarlenski MP, Wu AW, Bennett WL. Mammography use among women ages 40-49 after the 2009 U.S. Preventive Services Task Force recommendation. J Gen Intern Med. 2013;28(11):1447–53. doi:10.1007/s11606-013-2482-5.
Parris T, Wakefield D, Frimmer H. Real world performance of screening breast ultrasound following enactment of Connecticut Bill 458. Breast J. 19(1):64–70. doi: 10.1111/tbj.12053.
Rhodes DJ, Radecki Breitkopf C, Ziegenfuss JY, Jenkins SM, Vachon CM. Awareness of breast density and its impact on breast cancer detection and risk. J Clin Oncol. 2015;33(10):1143–50. doi:10.1200/JCO.2014.57.0325.
Acknowledgments
Dr. Richman was supported by a VA postdoctoral fellowship in health services research. Drs. Asch and Owens were supported by the Department of Veterans Affairs. Dr. Bhattacharya was supported by the National Institute on Aging grant R37-AG036791. This work was presented at the Society of General Internal Medicine Annual Meeting in Hollywood, Florida, May 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
This work does not necessarily represent the views of the Department of Veterans Affairs, and the authors are solely responsible for its content.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Richman, I., Asch, S.M., Bendavid, E. et al. Breast Density Notification Legislation and Breast Cancer Stage at Diagnosis: Early Evidence from the SEER Registry. J GEN INTERN MED 32, 603–609 (2017). https://doi.org/10.1007/s11606-016-3904-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-016-3904-y