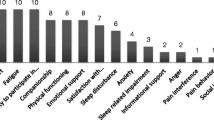
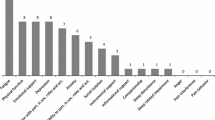
Context
Many treatments aim to improve patients’ health-related quality of life (HRQoL), and many care guidelines suggest assessing symptoms and their impact on HRQoL. However, there is a lack of consensus regarding which HRQoL outcome measures are appropriate to assess, and how much change on those measures depict significant HRQoL improvement.
Objective
We used triangulation methods to identify and understand clinically important differences (CIDs) for the amount of change in HRQoL that reflects both health professionals and patients’ values, among patients with chronic obstructive pulmonary disease (COPD).
Design, Setting, and Participants
We incorporated three perspectives: (1) an expert panel of physicians familiar with the measurement of HRQoL in COPD patients; (2) 610 primary care COPD outpatients who completed baseline and bimonthly follow-up HRQoL interviews over the 12-month study; and (3) the primary care physicians (PCPs; n = 43) of these outpatients who assessed their patients’ disease at baseline and at subsequent PCP visits during the year long study.
Measurements
The Chronic Respiratory Disease Questionnaire (CRQ), the Medical Outcomes Study Short Form 36-item survey (SF-36, version 2.0), and global assessments of change from each of the three perspectives for all HRQoL domains.
Results
With few exceptions, the CRQ was able to detect small changes at levels reported by the patients (1–2 points) and their PCPs (1–5 points). These results confirm minimal important difference standards developed in 1989 by Jaeschke et al. anchored on patient-perceived changes in HRQoL. In general, the expert panel and PCP CIDs were larger than the patient CIDs.
Conclusion
This triangulation methodology yielded improved interpretation, understanding, and insights on stakeholder perspectives of CIDs for patient-reported outcomes.





Similar content being viewed by others
References
Food and Drug Administration. Innovation or Stagnation: Challenges And Opportunities on the Critical Path to New Medical Products. Washington, DC: US Department of Health and Human Services; March 2004.
Schunemann H, Guyatt GH. Commentary—goodbye M(C)ID! Hello MID, where do you come from? Health Serv Res. 2005;40(2):593.
Jaeschke R, Singer J, Guyatt G. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.com; October 26, 2001.
Guyatt G, Berman L, Townsend M, Pugsley S, Chambers L. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–8.
Ware J, Kosinski M, Dewey J. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: QualityMetric Inc.; 2000.
Lacasse Y, Wong E, Guyatt G. A systematic overview of the measurement properties of the chronic respiratory questionnaire. Can Respir J. 1997;4(3):131–9.
Schunemann H, Griffith L, Jaeschke R, et al. A comparison of the original chronic respiratory questionnaire with a standardized version. Chest. 2003;124(4):1421–9.
Wyrwich K, Tierney W, Wolinsky F. Further evidence supporting a SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52(9):861–73.
Brazier J, Harper R, Jones N. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J. 1992;305:160–4.
Patrick D, Deyo R. Generic and disease-specific measures in assessing quality of life. Med Care. 1989;27:S217–32.
Wyrwich K, Fihn S, Tierney W, Kroenke K, Babu A, Wolinsky F. Clinically important differences in health-related quality of life for patients with chronic obstructive pulmonary disease: an expert panel report. J Gen Intern Med. 2003;18(3):196–202.
Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11:207–21.
Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40(2):577–92.
Curtis JR, Patrick DL. The assessment of health status among patients with COPD. Eur Respir J. 2003;21:36S–45S.
Webster GD. Final Report of the ABIM Patient Satisfaction Project. Philadelphia: American Board of Internal Medicine; 1988.
McHorney CA, Lerner J. The 1990 NORC National Health Survey: Documentation and Codebook. Chicago: National Opinion Research Council; 1991.
Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–14.
Mirowsky J, Ross CE. Eliminating defense and agreement bias from measures of the sense of control: a 2 × 2 index. Soc Psychol Q. 1991;54:127–45.
Fetzer Institute. Multidimensional Measurement of Religiousness/Spirituality for Use in Health Research. Kalamazoo: John E. Fetzer Institute; 1999.
Juniper E, Guyatt G, Willan A, Griffith L. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–7.
Statistical Package for the Social Sciences. SPSS for Windows, release 13.0.0. Chicago: SPSS, Inc.; 2005.
Streiner D, Norman G. Reliability. In: Health Measurement Scales, 3rd edn., Chap. 8. Oxford: Oxford University Press; 2003.
Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP-30. Qual Life Res. 2004;13:705–13.
Singh S, Sodergren S, Hyland M, Williams J, Morgan M. A comparison of three disease-specific and two generic health-status measures to evaluate the outcome of pulmonary rehabilitation in COPD. Respir Med. 2001;95:71–7.
Juniper E, Price D, Stampone P, Creemers J, Mol S, Fireman P. Clinically important improvements in asthma-specific quality of life, but no difference in conventional clinical indexes in patients changed from conventional beclomethasone dipropionate to approximately half the dose of extrafine beclomethasone dipropionate. Chest. 2002;121:1824–32.
Chauhan C, Eppard W, Perroti J. The patient advocate perspective on assessing the clinical significance for quality-of-life measures. J Cancer Integr Med. 2004;2(4):155–7.
Naylor CD, Llewellyn-Thomas HA. Can there be a more patient-centred approach to determining clinically important effect sizes for randomised trials? J Clin Epidemiol. 1994;47:787–95.
Norman G, Stratford P, Regehr G. Methodological problems in the retrospective computation of responsiveness to change: the lessons of Cronbach. J Clin Epidemiol. 1997;50(8):869–79.
Guyatt G, Norman G, et al. A critical look at transition ratings. J Clin Epidemiol. 2002;55:900–8.
Stratford P, Binkley J, Solomon P, et al. Defining the minimal level of detectable change for the Roland Morris Questionnaire. Phys Ther. 1996;76:359–65.
Schwartz C, Sprangers M. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48:1531–48.
Pulmonary Allergy Drugs Advisory Committee. New Drug Application (NDA) 21-395, Spiriva (Tiotropium bromide). Gaithersburg, MD: Food and Drug Administration; 2002.
Acknowledgement
This research was funded by grants from the Agency for Healthcare Research and Quality to Dr. Wolinsky (R01 HS10234) and Dr. Wyrwich (K02 HS11635).
Potential Financial Conflicts of Interest
None disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wyrwich, K.W., Metz, S.M., Kroenke, K. et al. Measuring Patient and Clinician Perspectives to Evaluate Change in Health-Related Quality of Life Among Patients with Chronic Obstructive Pulmonary Disease. J GEN INTERN MED 22, 161–170 (2007). https://doi.org/10.1007/s11606-006-0063-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-006-0063-6