Abstract
Background
Anastomotic leak (AL) is a feared complication after colorectal surgery. Prompt diagnosis and treatment are crucial. C-reactive protein (CRP) and procalcitonin (PCT) have been proposed as early AL indicators. The aim of this systematic review was to evaluate the CRP and CPT predictive values for early AL diagnosis after colorectal surgery.
Methods
Systematic literature search to identify studies evaluating the diagnostic accuracy of postoperative CRP and CPT for AL. A Bayesian meta-analysis was carried out using a random-effects model and pooled predictive parameters to determine postoperative CRP and PCT cut-off values at different postoperative days (POD).
Results
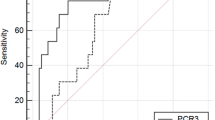
Twenty-five studies (11,144 patients) were included. The pooled prevalence of AL was 8% (95 CI 7–9%), and the median time to diagnosis was 6.9 days (range 3–10). The derived POD3, POD4 and POD5 CRP cut-off were 15.9 mg/dl, 11.4 mg/dl and 10.9 mg/dl respectively. The diagnostic accuracy was comparable with a pooled area under the curve (AUC) of 0.80 (95% CIs 0.23–0.85), 0.84 (95% CIs 0.18–0.86) and 0.84 (95% CIs 0.18–0.89) respectively. Negative likelihood ratios (LR−) showed moderate evidence to rule out AL on POD 3 (LR− 0.29), POD4 (LR− 0.24) and POD5 (LR− 0.26). The derived POD3 and POD5 CPT cut-off were 0.75 ng/ml (AUC = 0.84) and 0.9 ng/ml (AUC = 0.92) respectively. The pooled POD5 negative LR (−0.18) showed moderate evidence to rule out AL.
Conclusions
In the setting of colorectal surgery, CRP and CPT serum concentrations lower than the derived cut-offs on POD3-POD5, may be useful to rule out AL thus possibly identifying patients at low risk for AL development.








Similar content being viewed by others
References
Thornton M, Joshi H, Vimalachandran C, Heath R, Carter P, Gur U, Rooney P. Management and outcome of colorectal anastomotic leaks. Int J Colorectal Dis. 2011;26(3):313–20.
Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254–258.
Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999;189:554–559.
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102(5):462–79.
Sánchez–Guillén L, Frasson M, García–Granero Á, Pellino G, Flor–Lorente B, Álvarez–Sarrado E, García–Granero E. Risk factors for leak, complications and mortality after ileocolic anastomosis: comparison of two anastomotic techniques. Ann R Coll Surg Engl. 2019;101(8):571–578.
van Rooijen SJ, Huisman D, Stuijvenberg M, Stens J, Roumen RMH, Daams F, Slooter GD. Intraoperative modifiable risk factors of colorectal anastomotic leakage: Why surgeons and anesthesiologists should act together. Int J Surg. 2016;36(Pt A):183–200.
Huo YR, Phan K, Morris DL, Liauw W. Systematic review and a meta–analysis of hospital and surgeon volume/outcome relationships in colorectal cancer surgery. J Gastrointest Oncol. 2017;8(3):534–546.
Rausa E, Zappa MA, Kelly ME, Turati L, Russo L, Aiolfi A, Bonitta G, Sgroi LG (2019) A standardized use of intraoperative anastomotic testing in colorectal surgery in the new millennium: is technology taking over? A systematic review and network meta–analysis. Techniques in Coloproctology 23(7) 625–631. https://doi.org/10.1007/s10151-019-02034-6
Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, Bracale U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018;7;24(21):2247–2260.
Kastora SL, Osborne LL, Jardine R, Kounidas G, Carter B, Myint PK. Non–steroidal anti–inflammatory agents and anastomotic leak rates across colorectal cancer operations and anastomotic sites: A systematic review and meta–analysis of anastomosis specific leak rate and confounding factors. Eur J Surg Oncol. 2021;47(11):2841–2848.
Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, Fürst A, Lacy AM, Haglind E, Tuynman JB, Daams F, Bonjer HJ; COLOR COLOR II study group. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer: Increased Risk of Local Recurrence. Ann Surg. 2022;1;275(2):e420–e427.
Macarthur DC, Nixon SJ, Aitken RJ. Avoidable deaths still occur after large bowel surgery. Scottish Audit of Surgical Mortality, Royal College of Surgeons of Edinburgh. Br J Surg. 1998;85(1):80–3.
Aiolfi A, Bona B, Guerrazzi G, Bonitta G, Rausa E, Panizzo V, Campanelli G, Micheletto G (2020) Intracorporeal Versus Extracorporeal Anastomosis in Laparoscopic Right Colectomy: An Updated Systematic Review and Cumulative Meta–Analysis. Journal of Laparoendoscopic & Advanced Surgical Techniques. 30(4)402–412. https://doi.org/10.1089/lap.2019.0693
Lane JC, Wright S, Burch J, Kennedy RH, Jenkins JT. Early prediction of adverse events in enhanced recovery based upon the host systemic inflammatory response. Colorectal Dis. 2013;15(2):224–30.
Lagoutte N, Facy O, Ravoire A, Chalumeau C, Jonval L, Rat P, Ortega–Deballon P. C–reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg. 2012;149(5):e345–9.
Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, McMillan DC. C–reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19(13):4168–77.
Warschkow R, Beutner U, Steffen T, Muller SA, Schmied BM, Guller U et al. Safe and early discharge after colorectal surgery due to C–reactive protein: a diagnostic meta–analysis of 1832 patients. Ann Surg. 2012;256:245–250.
Bona D, Micheletto G, Bonitta G, Panizzo V, Cavalli M, Rausa E, Cirri S, Aiolfi A. Does C–reactive Protein Have a Predictive Role in the Early Diagnosis of Postoperative Complications After Bariatric Surgery? Systematic Review and Bayesian Meta–analysis. Obes Surg. 2019;29(11):3448–3456.
Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta–analysis of use of serum C–reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(4):339–46.
Facy O, Paquette B, Orry D, Binquet C, Masson D, Bouvier A, Fournel I, Charles PE, Rat P, Ortega–Deballon P; IMACORS Study. Diagnostic Accuracy of Inflammatory Markers As Early Predictors of Infection After Elective Colorectal Surgery: Results From the IMACORS Study. Ann Surg. 2016;263(5):961–6.
Sala Hernandez A, Frasson M, García–Granero A, Hervás Marín D, Laiz Marro B, Alonso Pardo R, Aldrey Cao I, Alvarez Perez JA, Roque Castellano C, García González JM, Tabet Almeida J, García–Granero E; EDEN study group. Diagnostic accuracy of C–reactive protein, procalcitonin and neutrophils for the early detection of anastomotic leakage after colorectal resection: a multicentric, prospective study. Colorectal Dis. 2021;23(10):2723–2730.
Yeung DE, Peterknecht E, Hajibandeh S, Hajibandeh S, Torrance AW. C–reactive protein can predict anastomotic leak in colorectal surgery: a systematic review and meta–analysis. Int J Colorectal Dis. 2021;36(6):1147–1162.
Adamina M, Steffen T, Tarantino I, Beutner U, Schmied BM, Warschkow R. Meta–analysis of the predictive value of C–reactive protein for infectious complications in abdominal surgery. Br J Surg. 2015;102(6):590–8.
Gans SL, Atema JJ, van Dieren S, Groot Koerkamp B, Boermeester MA. Diagnostic value of C–reactive protein to rule out infectious complications after major abdominal surgery: a systematic review and meta–analysis. Int J Colorectal Dis. 2015;30(7):861–73.
Cousin F, Ortega–Deballon P, Bourredjem A, Doussot A, Giaccaglia V, Fournel I. Diagnostic Accuracy of Procalcitonin and C–reactive Protein for the Early Diagnosis of Intra–abdominal Infection After Elective Colorectal Surgery: A Meta–analysis. Ann Surg. 2016 Aug;264(2):252–6.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta–analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. https://doi.org/10.1136/bmj.b2700.
Goossen K, Tenckhoff S, Probst P, et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg. 2018 Feb;403(1):119–129.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS–2 Group. QUADAS–2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;18;155(8):529–36.
Aiolfi A, Asti E, Rausa E, Bonavina G, Bonitta G, Bonavina L. Use of C–reactive protein for the early prediction of anastomotic leak after esophagectomy: Systematic review and Bayesian meta–analysis. PLoS One. 2018;17;13(12):e0209272.
Sutton AJ, Abrams KR. Bayesian methods in meta–analysis and evidence synthesis. Stat. Methods Med. Res. 2001;10:277–303.
Aiolfi A, Tornese S, Bonitta G, Rausa E, Micheletto G, Bona D. Roux–en–Y gastric bypass: systematic review and Bayesian network meta–analysis comparing open, laparoscopic, and robotic approach. Surg Obes Relat Dis. 2019;15(6):985–994.
Higgins JP, Thompson SG, Spiegelhalter DJ. A re–evaluation of random–effects meta–analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159.
Chu H, Cole SR. Bivariate meta–analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J Clin Epidemiol. 2006;59(12):1331–1332.
Wakefield J. Disease mapping and spatial regression with count data. Biostatistics. 2007;8(2):158–183.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta–analysis of diagnostic test accuracy evaluations. Statistics in Medicine. 2021;20(19):2865–2884.
Zwinderman AH, Bossuyt PM. We should not pool diagnostic likelihood ratios in systematic reviews. Stat Med. 2008;27(5):687–97.
Bona D, Lombardo F, Matsushima K, Cavalli M, Panizzo V, Mendogni P, Bonitta G, Campanelli G, Aiolfi A. Diaphragmatic herniation after esophagogastric surgery: systematic review and meta–analysis. Langenbecks Arch Surg. 2021;406(6):1819–1829.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing [cited 2022 Mar 14].
Kørner H, Nielsen HJ, Søreide JA, Nedrebø BS, Søreide K, Knapp JC. Diagnostic accuracy of C–reactive protein for intraabdominal infections after colorectal resections. J Gastrointest Surg. 2009;13(9):1599–606.
Garcia–Granero A, Frasson M, Flor–Lorente B, Blanco F, Puga R, Carratalá A, Garcia–Granero E. Procalcitonin and C–reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013;56(4):475–83.
Ramos Fernández M, Rivas Ruiz F, Fernández López A, Loinaz Segurola C, Fernández Cebrián JM, de la Portilla de Juan F. C reactive protein as a predictor of anastomotic leakage in colorectal surgery. Comparison between open and laparoscopic surgery. Cir Esp. 2017;95(9):529–535.
Reynolds IS, Boland MR, Reilly F, Deasy A, Majeed MH, Deasy J, Burke JP, McNamara DA. C–reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis. 2017;19(9):812–818.
Mik M, Dziki L, Berut M, Trzcinski R, Dziki A. Neutrophil to Lymphocyte Ratio and C–Reactive Protein as Two Predictive Tools of Anastomotic Leak in Colorectal Cancer Open Surgery. Dig Surg. 2018;35(1):77–84.
Zawadzki M, Krzystek–Korpacka M, Gamian A, Witkiewicz W. Serum cytokines in early prediction of anastomotic leakage following low anterior resection. Wideochir Inne Tech Maloinwazyjne. 2018;13(1):33–43.
Muñoz JL, Alvarez MO, Cuquerella V, Miranda E, Picó C, Flores R, Resalt–Pereira M, Moya P, Pérez A, Arroyo A. Procalcitonin and C–reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program. Surg Endosc. 2018;32(9):4003–4010.
Pantel HJ, Jasak LJ, Ricciardi R, Marcello PW, Roberts PL, Schoetz DJ Jr, Read TE. Should They Stay or Should They Go? The Utility of C–Reactive Protein in Predicting Readmission and Anastomotic Leak After Colorectal Resection. Dis Colon Rectum. 2019;62(2):241–247.
Pantoja Pachajoa DA, Gielis M, Palacios Huatuco RM, Benitez MN, Avila MN, Doniquian AM, Alvarez FA, Parodi M. Neutrophil–to–lymphocyte ratio vs C–reactive protein as early predictors of anastomotic leakage after colorectal surgery: A retrospective cohort study. Ann Med Surg (Lond). 2021;5;64:102201.
Baeza–Murcia M, Valero–Navarro G, Pellicer–Franco E, Soria–Aledo V, Mengual–Ballester M, Garcia–Marin JA, Betoret–Benavente L, Aguayo–Albasini JL. Early diagnosis of anastomotic leakage in colorectal surgery: prospective observational study of the utility of inflammatory markers and determination of pathological levels. Updates Surg. 2021;73(6):2103–2111.
Warschkow R, Steffen T, Beutner U, Müller SA, Schmied BM, Tarantino I. Diagnostic accuracy of C–reactive protein and white blood cell counts in the early detection of inflammatory complications after open resection of colorectal cancer: a retrospective study of 1,187 patients. Int J Colorectal Dis. 2012;27(10):1377.
Stephensen BD, Reid F, Shaikh S, Carroll R, Smith SR, Pockney P; PREDICT Study Group collaborators. C–reactive protein trajectory to predict colorectal anastomotic leak: PREDICT Study. Br J Surg. 2020;107(13):1832–1837.
Ortega–Deballon P, Radais F, Facy O, d'Athis P, Masson D, Charles PE, Cheynel N, Favre JP, Rat P. C–reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg. 2010;34(4):808–14.
Messias BA, Botelho RV, Saad SS, Mocchetti ER, Turke KC, Waisberg J. Serum C–reactive protein is a useful marker to exclude anastomotic leakage after colorectal surgery. Sci Rep. 2020;3;10(1):1687.
El Zaher HA, Ghareeb WM, Fouad AM, Madbouly K, Fathy H, Vedin T, Edelhamre M, Emile SH, Faisal M. Role of the triad of procalcitonin, C–reactive protein, and white blood cell count in the prediction of anastomotic leak following colorectal resections. World J Surg Oncol. 2022;12;20(1):33.
Jin D, Chen L. Early prediction of anastomotic leakage after laparoscopic rectal surgery using creactive protein. Medicine (Baltimore). 2021;4;100(22):e26196.
Almeida AB, Faria G, Moreira H, Pinto–de–Sousa J, Correia–da–Silva P, Maia JC. Elevated serum C–reactive protein as a predictive factor for anastomotic leakage in colorectal surgery. Int J Surg. 2012;10(2):87–91.
Waterland P, Ng J, Jones A, Broadley G, Nicol D, Patel H, Pandey S. Using CRP to predict anastomotic leakage after open and laparoscopic colorectal surgery: is there a difference? Int J Colorectal Dis. 2016 Apr;31(4):861–8.
Giaccaglia V, Salvi PF, Antonelli MS, Nigri G, Pirozzi F, Casagranda B, Giacca M, Corcione F, de Manzini N, Balducci G, Ramacciato G. Procalcitonin Reveals Early Dehiscence in Colorectal Surgery: The PREDICS Study. Ann Surg. 2016;263(5):967–72.
Italian ColoRectal Anastomotic Leakage (iCral) Study Group. Anastomotic leakage after elective colorectal surgery: a prospective multicentre observational study on use of the Dutch leakage score, serum procalcitonin and serum C–reactive protein for diagnosis. BJS Open. 2020;4(3):499–507.
Pepys MB, Hirschfield GM. C–reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–12.
Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta–analysis. Lancet Infect Dis. 2013;13(5):426–35.
Hamade B, Huang DT. Procalcitonin: Where Are We Now? Crit Care Clin. 2020;36(1):23–40.
Giaccaglia V, Salvi PF, Cunsolo GV, Sparagna A, Antonelli MS, Nigri G, Balducci G, Ziparo V. Procalcitonin, as an early biomarker of colorectal anastomotic leak, facilitates enhanced recovery after surgery. J Crit Care. 2014;29(4):528–32.
Sparreboom CL, Komen N, Rizopoulos D, Verhaar AP, Dik WA, Wu Z, van Westreenen HL, Doornebosch PG, Dekker JWT, Menon AG, Daams F, Lips D, van Grevenstein WMU, Karsten TM, Bayon Y, Peppelenbosch MP, Wolthuis AM, D'Hoore A, Lange JF. A multicentre cohort study of serum and peritoneal biomarkers to predict anastomotic leakage after rectal cancer resection. Colorectal Dis. 2020;22(1):36–45.
Akobeng AK. Understanding diagnostic tests 2: likelihood ratios, pre– and post–test probabilities and their use in clinical practice. Acta Paediatr. 2007;96(4):487–91.
Halkin A, Reichman J, Schwaber M, Paltiel O, Brezis M. Likelihood ratios: getting diagnostic testing into perspective. QJM. 1998;91(4):247–58.
Su'a B, Tutone S, MacFater W, Barazanchi A, Xia W, Zeng I, Hill AG. Diagnostic accuracy of procalcitonin for the early diagnosis of anastomotic leakage after colorectal surgery: a meta–analysis. ANZ J Surg. 2020;90(5):675–680.
Author information
Authors and Affiliations
Contributions
AA, AS, LC and MS did the literature search. AA and DB formed the study design. Data collection was done by AA, MS and FL. AA, GB, GC and DB analysed the data. AA, PD and DB interpreted the data. AA, GB, and DB wrote the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bona, D., Danelli, P., Sozzi, A. et al. C-reactive Protein and Procalcitonin Levels to Predict Anastomotic Leak After Colorectal Surgery: Systematic Review and Meta-analysis. J Gastrointest Surg 27, 166–179 (2023). https://doi.org/10.1007/s11605-022-05473-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05473-z