Abstract
Background
Gallbladder carcinoma (GBC) has a dismal prognosis even after curative resection. The objective of the study was to evaluate the effect of adjuvant chemotherapy in patients with GBC undergoing curative resection in a randomized control trial (RCT).
Methods
A single-center open-labeled prospective RCT was done from January 2012 to June 2018. R0 curative resected GBC patients were randomized in 1:1 to either surveillance alone (control group) or adjuvant chemotherapy (gemcitabine and cisplatin (GemCis group)) for 6 cycles. The primary outcome was disease-free survival (DFS), and the secondary outcomes were overall survival (OS) and toxicity profile.
Results
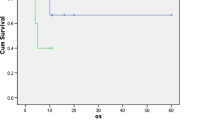
On the evaluation of 362 patients with GBC, 50 patients were enrolled in each control or GemCis group. Per protocol (PP), it comprised 96 patients. The demographic and clinical profile was similar between the two groups except in the lower nodal stage where patients were higher in the control group (p = 0.01). Recurrences were similar between groups (control 44% vs GemCis 56%; p = 0.23). On the intention to treat (ITT), analyses of median DFS (not reached vs. 24 months, p = 0.14) and OS (not reached vs. 31 months, p = 0.10) were similar between groups. On PP, analyses of median DFS (not reached vs. 24 months, p = 0.16) and OS (not reached vs. 31 months, p = 0.09) were similar between groups. The common toxicity profile was hematological followed by gastrointestinal symptoms.
Conclusions
Adjuvant GemCis therapy for 6 cycles does not improve DFS or OS than R0 surgery alone patients with GBC.
Trial Registration
NCT02778308 (https://www.clinicaltrials.gov)




Similar content being viewed by others
References
Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008 Dec 1;98(7):485–9.
Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klümpen H-J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer. 2015 Jul 31;15:564.
Kattepur AK, Patkar S, Goel M, Ramaswamy A, Ostwal V. Role of Adjuvant Chemotherapy in Resected T2N0 Gall Bladder Cancer. J Gastrointest Surg. 2019 Nov;23(11):2232–8.
Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003 Mar;4(3):167–76.
Choudhary S, Asthana AK. Impact of Adjuvant Therapy on Survival in Curatively Resected Gallbladder Carcinoma. J Clin Diagn Res. 2015 Sep;9(9):XC01–4.
6. Yasuda H, Takada T, Wada K, Amano H, Isaka T, Yoshida M, et al. A new in-vitro drug sensitivity test (collagen-gel droplet embedded-culture drug sensitivity test) in carcinomas of pancreas and biliary tract: possible clinical utility. J Hepatobiliary Pancreat Surg. 1998;5(3):261–8.
Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002 Oct 15;95(8):1685–95.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010 Apr 8;362(14):1273–81.
Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study – The UK ABC-01 Study. Br J Cancer. 2009 Aug 18;101(4):621–7.
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010 Aug 10;103(4):469–74.
Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012 Jun 1;30(16):1934–40.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly J-P, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019 Mar 10;37(8):658–67.
Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019 May;20(5):663–73.
Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015 Aug 20;33(24):2617–22.
Bergquist JR, Shah HN, Habermann EB, Hernandez MC, Ivanics T, Kendrick ML, et al. Adjuvant systemic therapy after resection of node positive gallbladder cancer: Time for a well-designed trial? (Results of a US-national retrospective cohort study). Int J Surg. 2018 Apr;52:171–9.
Ma N, Cheng H, Qin B, Zhong R, Wang B. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 2015 Sep 3;15(1):615.
Manterola C, Duque G, Grande L, Aretxabala X de, Conejeros R, Otzen T, et al. A systematic review of the effectiveness of adjuvant therapy for patients with gallbladder cancer. HPB. 2019 Nov 1;21(11):1427–35.
Tran Cao HS, Zhang Q, Sada YH, Chai C, Curley SA, Massarweh NN. The role of surgery and adjuvant therapy in lymph node-positive cancers of the gallbladder and intrahepatic bile ducts. Cancer. 2018 Jan 1;124(1):74–83.
Go S-I, Kim YS, Hwang IG, Kim EY, Oh SY, Ji JH, et al. Is There a Role for Adjuvant Therapy in R0 Resected Gallbladder Cancer?: A Propensity Score-Matched Analysis. Cancer Res Treat. 2016 Oct;48(4):1274–85.
Chen C, Feng W, Zheng Y, Bao Y, Feng M. Intra-arterial chemotherapy improved survival of stage 2–3 gallbladder cancer after curative resection. Onco Targets Ther. 2018 May 22;11:2975–9.
Kasumova GG, Tabatabaie O, Najarian RM, Callery MP, Ng SC, Bullock AJ, et al. Surgical Management of Gallbladder Cancer: Simple Versus Extended Cholecystectomy and the Role of Adjuvant Therapy. Ann Surg. 2017 Oct;266(4):625–31.
Kim TG. Patterns of initial failure after resection for gallbladder cancer: implications for adjuvant radiotherapy. Radiat Oncol J. 2017 Dec;35(4):359–67.
Nakamura M, Nakashima H, Abe T, Ensako T, Yoshida K, Hino K. Gemcitabine-based adjuvant chemotherapy for patients with advanced gallbladder cancer. Anticancer Res. 2014 Jun;34(6):3125–9.
ECOG-ACRIN Cancer Research Group. Optimal Perioperative Therapy for Incidental Gallbladder Cancer (OPT-IN): A Randomized Phase II/III Trial [Internet]. clinicaltrials.gov; 2020 Dec [cited 2021 Feb 11]. Report No.: NCT04559139. Available from: https://clinicaltrials.gov/ct2/show/NCT04559139
Sharma A, Shukla NK, Chaudhary SP, Sahoo R, Mohanti B, Deo SVS, et al. Final results of a phase III randomized controlled trial comparing modified gemcitabine + oxaliplatin (mGEMOX) to gemcitabine+ cisplatin in management of unresectable gall bladder cancer (GBC). JCO. 2016 May 20;34(15_suppl):4077–4077.
Author information
Authors and Affiliations
Contributions
All the authors have made substantial contributions to the conception or design of the work; the acquisition, analysis, and interpretation of data; and drafting of the work or revising it critically for important intellectual content.
The final approvers of the version to be published are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saluja, S.S., Nekarakanti, P.K., Mishra, P.K. et al. Prospective Randomized Controlled Trial Comparing Adjuvant Chemotherapy vs. No Chemotherapy for Patients with Carcinoma of Gallbladder Undergoing Curative Resection. J Gastrointest Surg 26, 398–407 (2022). https://doi.org/10.1007/s11605-021-05143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05143-6