Abstract
Introduction
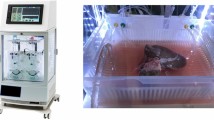
The small intestine is one of the most ischemia-sensitive organs used in transplantation. To better preserve the intestinal graft viability and decrease ischemia-reperfusion injury, a device for extracorporeal perfusion was developed. We present the results for the first series of perfused human intestine with an intestinal perfusion unit (IPU).
Methods
Five human intestines were procured for the protocol. (1) An experimental segment was perfused by the IPU delivering cold preservation solution to the vascular and luminal side continually at 4 ºC for 8 h. (2) Control (jejunum and ileum) segments were preserved in static cold preservation. Tissue samples were obtained for histopathologic grading according to the Park/Chiu scoring system (0 = normal, 8 = transmural infarction).
Results
Jejunal experimental segments scored 2.2 with the Park/Chiu system compared to the control segments, which averaged 3.2. Overall scoring for ileum experimental and control segments was equal with 1.6.
Conclusion
This data presents proof of concept that extracorporeal intestinal perfusion is feasible. The evidence shows that the IPU can preserve the viability of human intestine, and histopathologic evaluation of perfused intestine is favorable. Our early results can eventually lead to expanding the possibilities of intestinal preservation.




Similar content being viewed by others
References
Grant, D., et al., Successful small-bowel/liver transplantation. The Lancet, 1990. 335(8683): p. 181–184.
Fishbein, T.M., Intestinal transplantation. N Engl J Med, 2009. 361(10): p. 998–1008.
Reyes, J.D., Intestinal transplantation: an unexpected journey. Robert E. Gross Lecture. J Pediatr Surg, 2014. 49(1): p. 13–8.
OPTN. U.S. Intestinal Transplants Performed: January 1, 1995—January 31,2014. 2014 [cited 2015; Available from: http://optn.transplant.hrsa.gov/.
Smith, J., et al., OPTN/SRTR 2013 Annual Data Report: Intestine. American Journal of Transplantation, 2015. 15(S2): p. 1–16.
Moers, C., et al., Machine perfusion or cold storage in deceased-donor kidney transplantation. New England Journal of Medicine, 2009. 360(1): p. 7–19.
St Peter, S., et al., Extended preservation of non‐heart‐beating donor livers with normothermic machine perfusion. British journal of surgery, 2002. 89(5): p. 609–616.
Cobert, M.L., L.M. West, and M.E. Jessen, Machine perfusion for cardiac allograft preservation. Current opinion in organ transplantation, 2008. 13(5): p. 526–530.
Cypel, M., et al., Normothermic ex vivo lung perfusion in clinical lung transplantation. New England Journal of Medicine, 2011. 364(15): p. 1431–1440.
Henry, S.D. and J.V. Guarrera, Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando), 2012. 26(2): p. 163–75.
Petrowsky, H., Organ procurement and preservation: what is new and what is established? Current opinion in organ transplantation, 2014. 19(2): p. 83–84.
Cameron, A.M. and J.F.B. Cornejo, Organ preservation review: history of organ preservation. Current opinion in organ transplantation, 2015. 20(2): p. 146–151.
Oltean, M., Intestinal preservation for transplantation: current status and alternatives for the future. Curr Opin Organ Transplant, 2015. 20(3): p. 308–13.
Abu-Elmagd, K., et al., Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Annals of surgery, 2000. 232(5): p. 680.
Casavilla, A., et al., Logistics and technique for combined hepatic-intestinal retrieval. Annals of surgery, 1992. 216(5): p. 605.
Yersiz, H., et al., Multivisceral and isolated intestinal procurement techniques. Liver transplantation, 2003. 9(8): p. 881–886.
Leuvenink, H.G., et al., Luminal preservation of rat small intestine with University of Wisconsin or Celsior solution. Transplant Proc, 2005. 37(1): p. 445–7.
Oltean, M., et al., Intraluminal polyethylene glycol stabilizes tight junctions and improves intestinal preservation in the rat. Am J Transplant, 2012. 12(8): p. 2044–51.
Toledo-Pereyra, L.H. and J.S. Najarian, Small bowel preservation. Comparison of perfusion and nonperfusion systems. Arch Surg, 1973. 107(6): p. 875–7.
Toledo-Pereyra, L.H., R.L. Simmons, and J.S. Najarian, Two-to-three day intestinal preservation utilizing hypothermic pulsatile perfusion. Ann Surg, 1974. 179(4): p. 454–9.
Toledo-Pereyra, L.H. and J.S. Najarian, Human small bowel preservation: assessment of viability during storage. Bol Asoc Med P R, 1979. 71(9): p. 336–41.
Zhu, J.Z., et al., A novel technique of hypothermic luminal perfusion for small bowel preservation. Transplantation, 2003. 76(1): p. 71–6.
Balaz, P., et al., Preservation injury of the small bowel graft in clinical small bowel transplantation. Bratisl Lek Listy, 2007. 108(12): p. 516–8.
Kato, T., et al., Intestinal and multivisceral transplantation. World journal of surgery, 2002. 26(2): p. 226–237.
Brockmann, J., et al., Normothermic perfusion: a new paradigm for organ preservation. Ann Surg, 2009. 250(1): p. 1–6.
Fuller, B.J. and D.E. Pegg, The assessment of renal preservation by normothermic bloodless perfusion. Cryobiology, 1976. 13(2): p. 177–84.
St Peter, S.D., C.J. Imber, and P.J. Friend, Liver and kidney preservation by perfusion. The Lancet, 2002. 359(9306): p. 604–613.
Fraczek, M., et al., Small bowel transplantation--harvesting technique and graft preparation in pigs. Ann Transplant, 2007. 12(1): p. 19–26.
Yandza, T., et al., The pig as a preclinical model for intestinal ischemia-reperfusion and transplantation studies. J Surg Res, 2012. 178(2): p. 807–19.
Narayan, R.R., et al., A novel device to preserve intestinal tissue ex-vivo by cold peristaltic perfusion. Conf Proc IEEE Eng Med Biol Soc, 2014. 2014: p. 3118–21.
Agarwal, A., et al. Comparison of histidine-tryptophan ketoglutarate and University of Wisconsin solutions as primary preservation in renal allografts undergoing pulsatile perfusion. in Transplantation proceedings. 2005. Elsevier.
Southard, M., James H and M. Belzer, Folkert O, Organ preservation. Annual review of medicine, 1995. 46(1): p. 235–247.
DeRoover, A., et al., Luminal contact with University of Wisconsin solution improves human small bowel preservation. Transplantation Proceedings, 2004. 36(2): p. 273–275.
Pienaar, B.H., et al., Seventy-two-hour preservation of the canine liver by machine perfusion. Transplantation, 1990. 49(2): p. 258–60.
Geibel, J.P. and S.C. Hebert, The functions and roles of the extracellular Ca2 + −sensing receptor along the gastrointestinal tract. Annual Review of Physiology, 2009. 71: p. 205–217.
D’Amico, F., et al., Use of N‐acetylcysteine during liver procurement: A prospective randomized controlled study. Liver Transplantation, 2013. 19(2): p. 135–144.
Guarrera, J.V., et al., A novel ex-vivo porcine renal xenotransplantation model using a pulsatile machine preservation system. Ann Transplant, 2011. 16(1): p. 80–2.
Abdal Raheem, S., et al., Bariatric surgery complications leading to small bowel transplant: a report of 4 cases. JPEN J Parenter Enteral Nutr, 2014. 38(4): p. 513–7.
Corcos, O., et al., Intestinal failure after bariatric surgery. Lancet, 2013. 382(9893): p. 742.
McBride, C.L., et al., Short bowel syndrome following bariatric surgical procedures. Am J Surg, 2006. 192(6): p. 828–32.
Acknowledgments
The authors of this article acknowledge the hard work and contribution of Natalie E. Pancer, Brian W. Loeb, Kristi E. Oki, Andrew Crouch, Yusuf Chauhan, Spencer Backus, and Richard E. Fan on the early stages of this project.
This project was performed in collaboration with Life Choice of Connecticut, to whom we are grateful for their support.
This research was funded by the Ohse grants from the Department of Surgery Yale University and the CBIT grants program at the Yale University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant Support
Ohse Grants 2014–2015—Department of Surgery, Yale School of Medicine, New Haven, CT.
CBIT Grants 2015—Yale School of Medicine, New Haven, CT
Additional information
Primary Discussant
Sean P Harbison M.D. (Philadelphia, PA)
Organ transplantation for intestinal failure is labor and resource intensive. Among the many formidable limitations and obstacles facing those groups who perform the hundred-odd small bowel transplants annually, ischemia/reperfusion injury may be the most imposing and rate limiting. The authors are to be applauded for taking on a seeming Herculean task; applying sound reductionist scientific principles and multidisciplinary cooperation, they have developed an elegantly simple, workable intestinal perfusion unit (IPU) providing both luminal and vascular perfusate. Tested (remarkably) on human small bowel preparations, this study essentially represents Phase I or Proof of Concept with the additional positive outcome of suggestion of efficacy. The small number of small bowel preparations studied (N = 5) is statistically limiting but should not be considered a major flaw since the authors have shown that an IPU is feasible and may indeed mitigate ischemic injury. The obvious question to the authors is which direction next? The IPU and small bowel model they have developed might be used to answer a multitude of questions. What is the optimal perfusate? Which temperature is optimal? Is there an oxygen- carrying capability or a metabolite-scavenging one? Can the physical environment provided by the IPU itself be optimized, for example, might the rate of perfusion be altered or perhaps the manner of perfusion such as pulsatile flow in order to minimize ischemia? I congratulate the authors for their accomplishment: this project clearly entailed much hard work which may not be manifested by their fundamental and simple solution. It is most often the simple solution that is the best.
I thank the authors for the privilege of discussing this paper.
Closing Discussant
Dr. Munoz-Abraham
Thank you very much for your insightful comments. We too find this project to be an exciting endeavor. To answer your questions, the direction of this project is to increase the n by obtaining additional specimens, thus reaching statistical significance and adding power to our study. Also, we plan to increase the time that each specimen remains on the IPU in hopes of further demonstrating that perfusion gives increased survival characteristics. We are still unsure what the final optimal perfusate is. We have been working with both UW and HTK as these are well-known and tested hypothermic solutions for other organs. The question for temperature and additional components is a great, and by no means, an easy one to answer. The ideal temperature (hypothermic, normothermic, or subnormothermic) for intestinal preservation ex vivo is still a mystery and is a very interesting area that we are looking into exploring in the near future. For our current device, we have not added oxygen-carrying capacity for our hypothermic solutions, but if normothermic preservation is to be explored, we will investigate the options for oxygenation and other modifications as outlined above. Regarding the quality of perfusion, pulsatile versus continuous, this is another excellent question; we believe that the modular design of our device allows versatility for exchanging the pump head(s) to provide both types of perfusion and even a combination, if desired, for lumen and vasculature.
Again, we feel very grateful for having you as our discussant and look forward to continued discussions together in the future.
Rights and permissions
About this article
Cite this article
Muñoz-Abraham, A.S., Patrón-Lozano, R., Narayan, R.R. et al. Extracorporeal Hypothermic Perfusion Device for Intestinal Graft Preservation to Decrease Ischemic Injury During Transportation. J Gastrointest Surg 20, 313–321 (2016). https://doi.org/10.1007/s11605-015-2986-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2986-x