Abstract
Background
The aims of this study were to investigate metastasis suppressor 1 (MTSS1) expression in benign and malignant colorectal tissues and to explore its significance in the prognosis of colorectal cancer (CRC) patients.
Methods
MTSS1 expression was detected by immunohistochemistry in CRC, colorectal adenomatous polyp (precancerous lesion) and normal colorectal tissues. The relationship between MTSS1 expression in CRC tissues and clinicopathologic factors was analyzed with Mann–Whitney U test. MTSS1 protein expression was observed by Western blot in CRC tissues and adjacent nontumor colorectal tissues. Two factors between MTSS1 expression and CRC patient tumor node metastasis (TNM) stage were analyzed by Spearman rank correlation analysis. The Kaplan–Meier method and log-rank test were employed to compare the overall survival between MTSS1 negative/weak positive expression group and MTSS1 strong positive expression group.
Results
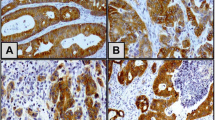
MTSS1 expression rates were significantly higher in CRC tissues (99 out of 135, 73.30%) than that in normal colorectal tissues (one out of seven, 14.29%), nontumor colorectal tissues (six out of 32, 18.75%), and adenomatous polyp tissues (four out of 15, 26.67%; P = 0.003, P < 0.001, P = 0.001, respectively). The upregulated MTSS1 expression in CRC tissues was significantly correlated to poor differentiation (P = 0.005), tissue invasion (P = 0.018), high preoperative CEA level (P = 0.022), present lymph node metastasis (P = 0.003), and high TNM stage (P = 0.002). MTSS1 expression was positively correlated with clinical TNM stage, that suggested the more advanced clinical TNM stage corresponding to the higher expression level of MTSS1 (r s = 0.327, P < 0.05). Western blotting demonstrated that MTSS1 expression was upregulated in 25 of 32 CRC tissues (75.0%) compared to corresponding adjacent nontumor colorectal tissues. The overall 5-year survival of MTSS1 strong positive expression CRC patients was significantly shorter than that of MTSS1 negative and weakly positive expression group. In multivariate analysis, MTSS1 expression maintained independent prognostic influence on overall survival (P = 0.004).
Conclusion
MTSS1 may be a good biomarker to be applied in the clinical setting to predict the prognosis of CRC patients with completely resected.



Similar content being viewed by others
References
Sung JJ, Lau JY, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6(11):871-6.
Lee YG, Macoska JA, Korenchuk S, et al. MIM, a potential metastasis suppressor gene in bladder cancer. Neoplasia. 2002; 4(4): 291–4.
Lin J, Liu J, Wang Y, et al. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene. 2005; 24(12): 2059–66.
Yamagishi A, Masuda M, Ohki T, et al. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J Biol Chem. 2004;279(15):14929–36.
Loberg RD, Neeley CK, Adam-Day LL, et al. Differential expression analysis of M IM (MTSS1) sp lice variants and a functional role of MIM in prostate cancer cell biology. Int J Oncol. 2005; 26(6):1699–1705
Wang Y, Liu J, Smith E, et al. Downregulation ofmissing in metastasis gene (MIM) is associated with the p rogression of bladder transitional carcinomas. Cancer Invest. 2007; 25(2):79–86.
Wang Y, Zhou K, Zeng X, et al. Tyrosine phosphorylation of missing in metastasis protein is implicated in platelet-derived growth factor-mediated cell shape changes. J Biol Chem. 2007; 282(10):7624–7631.
Lee YY, Yu CP, Lin CK, et al. Expression of survivin and cortactin in colorectal adenocarcinoma: Association with clinicopathological parameters. Dis Markers 2009;26:9–18.
Nixdorf S, Grimm MO, Loberg R, et al. Expression and regulation of MIM (Missing in Metastasis), a novel putative metastasis suppressor gene, and MIM-B, in bladder cancer cell lines. Cancer Lett. 2004;215:209–22.
Parr C, Jiang WG. Metastasis suppressor 1 (MTSS1) demonstrates prognostic value and anti-metastatic properties in breast cancer. Eur J Cancer. 2009;45(9):1673–83.
Liu K, Wang GF, Ding HZ, et al. Downregulation of metastasis suppressor 1 (MTSS1) is associated with nodal metastasis and poor outcome in Chinese patients with gastric cancer. BMC Cancer. 2010; 10:428–37.
Callahan CA, Ofstad T, Horng L, et al. MIM/BEG4, a Sonic hedgehog-responsive gene that potentiates Gli-dependent transcription. Genes Dev. 2004; 18(22): 2724–9.
Woodings JA, Sharp SJ, Machesky LM. MIM-B, a putative metastasis suppressor protein, binds to actin and to protein tyrosine phosphatase delta. Biochem J. 2003;371:463–71.
Utikal J, Gratchev A, Muller-Molinet I, et al. The expression of metastasis suppressor MIM/MTSS1 is regulated by DNA methylation. Int J Cancer. 2006; 119(10):2287–2293.
Huang XY, Huang ZL, Tang ZY, et al. Experimental observation on the alteration of metastatic potential and its gene function net of residual hepatocellular carcinoma following palliative liver resection. Zhonghua Yi Xue Za Zhi. 2010;90(18):1278–82.
Ma S, Guan XY, Lee TK, et al. Clinicopathological significance of missing in metastasis B expression in hepatocellular carcinoma. Hum Pathol. 2007; 38(8): 1201–6.
Gonzalez-Quevedo R, Shoffer M, Horng L, et al. Receptor tyrosine phosphatase-dependent cytoskeletal remodeling by the hedgehog-responsive gene MIM/BEG4. J Cell Biol.2005. 168(3):453–63.
Bershteyn M, Atwood SX, Woo WM, et al. MIM and Cortactin Antagonism Regulates Ciliogenesis and Hedgehog Signaling. Dev Cell. 2010;19(2):270–83.
Acknowledgments
We wish to thank two pathologists, Prof. Hua Huang and Prof. Jian-guo Zhang for their technical assistance. The research was supported by the National Natural Science Foundation of China (No. 30771126).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Xu, Mr., Wang, T. et al. MTSS1 Overexpression Correlates with Poor Prognosis in Colorectal Cancer. J Gastrointest Surg 15, 1205–1212 (2011). https://doi.org/10.1007/s11605-011-1546-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1546-2