Abstract
Introduction
Intestinal epithelial cells represent an important component of innate immunity, with sophisticated responses to inflammatory stimuli. The manner in which intestinal epithelial cell polarity affects responses to inflammatory stimuli is largely unknown. We hypothesized that polarized intestinal epithelial cells exhibit a bidirectional inflammatory response dependent upon the location of the stimulus.
Methods
Caco-2 cells were grown on semi-permeable inserts in a dual-compartment culture system and treated with tumor necrosis factor-α (TNF-α; 100 ng/ml) or serum-free media in the apical or basolateral chamber. Interleukin-8 (IL-8) production in each chamber was measured by enzyme-linked immunosorbent assay. To determine receptor specificity, anti-TNF receptor antibodies were added to the apical or basolateral chamber.
Results
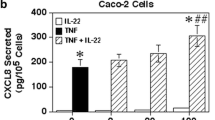
Basolateral stimulation with TNF-α resulted in increased apical and basolateral IL-8 production. Apical TNF-α stimulation resulted in increased apical, but not basolateral IL-8 production. Receptor blockade suggested TNF receptor 1 involvement on both apical and basolateral membranes, while TNF receptor 2 was only active on the apical membrane.
Conclusion
Polarized intestinal epithelial cells respond to TNF-α stimulation with focused, directional secretion of the proinflammatory cytokine IL-8. These findings are important because they suggest that intestinal epithelial cells are capable of organizing their response to inflammatory signals and producing inflammatory mediators in a bidirectional, vectorial fashion.






Similar content being viewed by others
References
Gosain, A. and R.L. Gamelli, Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil, 2005. 26(1): p. 85–91.
Diebel, L.N. and D.M. Liberati, Intestinal epithelial cells mediate lung injury after ethanol exposure and hypoxic insult. J Trauma, 2009. 67(2): p. 296–302.
Clark, J.A. and C.M. Coopersmith, Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock, 2007. 28(4): p. 384–93.
Lenz, A., G.A. Franklin, and W.G. Cheadle, Systemic inflammation after trauma. Injury, 2007. 38(12): p. 1336–45.
Rogler, G. and T. Andus, Cytokines in inflammatory bowel disease. World J Surg, 1998. 22(4): p. 382–9.
Alverdy, J.C. and E.B. Chang, The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol, 2008. 83(3): p. 461–6.
McCormick, B.A., et al., Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol, 1998. 160(1): p. 455–66.
Neal, M.D., et al., Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol, 2006. 176(5): p. 3070–9.
Reilly, N., et al., Probiotics potentiate IL-6 production in IL-1beta-treated Caco-2 cells through a heat shock-dependent mechanism. Am J Physiol Regul Integr Comp Physiol, 2007. 293(3): p. R1169–79.
Wehkamp, J., et al., NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun, 2004. 72(10): p. 5750-8.
Wu, L., et al., Recognition of host immune activation by Pseudomonas aeruginosa. Science, 2005. 309(5735): p. 774–7.
Bradley, J.R., TNF-mediated inflammatory disease. J Pathol, 2008. 214(2): p. 149–60.
Wajant, H., K. Pfizenmaier, and P. Scheurich, Tumor necrosis factor signaling. Cell Death Differ, 2003. 10(1): p. 45–65.
Spies, M., et al., Role of TNF-alpha in gut mucosal changes after severe burn. Am J Physiol Gastrointest Liver Physiol, 2002. 283(3): p. G703–8.
Colombel, J.F., et al., Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med., 2010. 362(15): p. 1383–95.
Owczarek, D., et al., Biological therapy of inflammatory bowel disease. Pol Arch Med Wewn, 2009. 119(1–2): p. 84–8.
Appau, K.A., et al., Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn’s patients. J Gastrointest Surg, 2008. 12(10): p. 1738–44.
Marehbian, J., et al., Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol, 2009. 104(10): p. 2524–33.
Tamion, F., et al., Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol, 1997. 273(2 Pt 1): p. G314–21.
Onizawa, M., et al., Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol, 2009. 296(4): p. G850–9.
Bocker, U., et al., Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis, 2003. 18(1): p. 25–32.
Holtkamp, G.M., et al., Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol, 1998. 112(1): p. 34–43.
Cudicini, C., et al., Vectorial production of interleukin 1 and interleukin 6 by rat Sertoli cells cultured in a dual culture compartment system. Endocrinology, 1997. 138(7): p. 2863–70.
Kruger, S., et al., Interleukin-8 secretion of cortical tubular epithelial cells is directed to the basolateral environment and is not enhanced by apical exposure to Escherichia coli. Infect Immun, 2000. 68(1): p. 328–34.
Panigrahi, P., et al., Escherichia coli transcytosis in a Caco-2 cell model: implications in neonatal necrotizing enterocolitis. Pediatr Res, 1996. 40(3): p. 415–21.
Nasreen, N., et al., Polar production of interleukin-8 by mesothelial cells promotes the transmesothelial migration of neutrophils: role of intercellular adhesion molecule-1. J Infect Dis, 2001. 183(11): p. 1638–45.
Sitaraman, S.V., et al., Neutrophil–epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest, 2001. 107(7): p. 861–9.
Vreugdenhil, A.C., et al., Lipopolysaccharide-binding protein is vectorially secreted and transported by cultured intestinal epithelial cells and is present in the intestinal mucus of mice. J Immunol, 2000. 165(8): p. 4561–6.
Zeillemaker, A.M., et al., Polarized secretion of interleukin-8 by human mesothelial cells: a role in neutrophil migration. Immunology, 1995. 84(2): p. 227–32.
Chantret, I., et al., Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res, 1988. 48(7): p. 1936–42.
Grasset, E., J. Bernabeu, and M. Pinto, Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol, 1985. 248(5 Pt 1): p. C410–8.
Grasset, E., et al., Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol, 1984. 247(3 Pt 1): p. C260–7.
Abreu, M.T., M. Fukata, and M. Arditi, TLR signaling in the gut in health and disease. J Immunol, 2005. 174(8): p. 4453–60.
Duizer, E., et al., Absorption enhancement, structural changes in tight junctions and cytotoxicity caused by palmitoyl carnitine in Caco-2 and IEC-18 cells. J Pharmacol Exp Ther, 1998. 287(1): p. 395–402.
Li, N., et al., Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem, 2003. 14(7): p. 401–8.
Baud, V. and M. Karin, Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol, 2001. 11(9): p. 372–7.
Ferrero, E., et al., Roles of tumor necrosis factor p55 and p75 receptors in TNF-alpha-induced vascular permeability. Am J Physiol Cell Physiol, 2001. 281(4): p. C1173–9.
Orlinick, J.R. and M.V. Chao, TNF-related ligands and their receptors. Cell Signal, 1998. 10(8): p. 543–51.
Edelblum, K.L., et al., TNFR1 promotes tumor necrosis factor-mediated mouse colon epithelial cell survival through RAF activation of NF-kappaB. J Biol Chem, 2008. 283(43): p. 29485–94.
Heyninck, K. and R. Beyaert, Crosstalk between NF-kappaB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol Cell Biol Res Commun, 2001. 4(5): p. 259–65.
Neurath, M.F., C. Becker, and K. Barbulescu, Role of NF-kappaB in immune and inflammatory responses in the gut. Gut, 1998. 43(6): p. 856–60.
Holtmann, M.H. and M.F. Neurath, Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med, 2004. 4(4): p. 439–44.
Vallee, S., et al., Cytokine-induced upregulation of NF-kappaB, IL-8, and ICAM-1 is dependent on colonic cell polarity: implication for PKCdelta. Exp Cell Res, 2004. 297(1): p. 165–85.
Gross, V., et al., Regulation of interleukin-8 production in a human colon epithelial cell line (HT-29). Gastroenterology, 1995. 108(3): p. 653–61.
Schroder, J.M. and E. Christophers, Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human endothelial cells. J Immunol, 1989. 142(1): p. 244–51.
Strieter, R.M., et al., Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem, 1989. 264(18): p. 10621–6.
Kucharzik, T., et al., Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut, 2005. 54(11): p. 1565–72.
McCormick, B.A., Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. FEBS J, 2007. 274(14): p. 3513–8.
Blake, K.M., et al., Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol, 2004. 136(2): p. 262–8.
Kim, J.M., et al., Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol, 2002. 130(1): p. 59–66.
Kucharzik, T. and I.R. Williams, Neutrophil migration across the intestinal epithelial barrier—summary of in vitro data and description of a new transgenic mouse model with doxycycline-inducible interleukin-8 expression in intestinal epithelial cells. Pathobiology, 2002. 70(3): p. 143–9.
Mammen, J.M. and J.B. Matthews, Mucosal repair in the gastrointestinal tract. Crit Care Med, 2003. 31(8 Suppl): p. S532–7.
Maheshwari, A., et al., Effects of interleukin-8 on the developing human intestine. Cytokine, 2002. 20(6): p. 256–67.
Sturm, A., et al., CXCL8 modulates human intestinal epithelial cells through a CXCR1 dependent pathway. Cytokine, 2005. 29(1): p. 42–8.
Heidemann, J., et al., Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem, 2003. 278(10): p. 8508–15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Edward E. Whang (Boston, MA): Good job with the studies and very clear presentation. TNF-stimulated IL-8 secretion in this cell line is a well-known phenomenon. Your identification of directional asymmetry to this process is intriguing. I will ask you to address issues related to validity and biological significance of these findings.
First, you have suggested but not actually shown differential distribution of TNF receptor subtypes. Have you done these studies?
Second, Caco-2 cells are commonly used to model small intestinal epithelium, but they are colon cancer cells, after all. Are you aware whether normal small bowel enterocytes express TNF receptors and whether they respond to TNF by secreting IL-8? How would you validate that the directionality of TNF-stimulated IL-8 secretion you have reported today exists in normal intestine?
Finally, let us assume you were to figure out the detailed mechanisms responsible for this directional phenomenon. What would you do with that information? What would be the potential biological or clinical significance of this information?
Closing Discussant
Dr. Dennis Sonnier: Thank you very much for your comments and insightful questions. I think that looking at this in an ex vivo setting or in other cell lines would be helpful.
In addition, the IBD literature contains data regarding expression of various inflammatory markers in the stool, and they use this to track disease. This suggests that findings similar to ours make occur in the in vivo setting. IBD patients also show considerable responses to anti-TNF receptor therapy. The presence and function of these receptors on the apical membrane in tissue specimens and animal models is something else we plan on looking at as well.
What do we plan to do with this? Transferring this into a mouse model after we work out some of the cellular mechanisms will be going to be very important for studying various causes of intestinal inflammation as well as possible use in assessment of clinical severity of intestinal inflammation after trauma.
Regarding your questions about receptor expression, I agree that we have not demonstrated receptor expression during these studies. We have performed some initial confocal microscopy studies to help determine receptor expression.
Discussant
Dr. Michael Sarr (Rochester, MN): There are several other enterocyte-like cell models, such as RIE and IEC-6 cells. And they are more from younger animals. Have you thought about looking at those cell lines as well?
Closing Discussant
Dr. Dennis Sonnier: Thank you for your comments and questions. We have not yet looked at those specific cell lines, but these and other cell lines should be considered. We used Caco-2 cells in the current experiments because of their known ability to polarize when differentiated on Transwells.
Discussant
Dr. Michael Sarr (Rochester, MN): Why would a cell secrete something into the lumen when it is polarized. We have been struggling with that, looking at the effects of some hormones that are secreted into the lumen that have an effect. Why does this cell line secrete IL-8 into the lumen?
Closing Discussant
Dr. Dennis Sonnier: Dr. Sarr, that is a great question. By way of pure speculation, I think luminal secretion of cytokines may be a way of autocrine or paracrine control of inflammation. Upstream, intestinal epithelial cells can control a response downstream by the mediators that they secrete into the lumen in a way that cannot be achieved by secreting basolaterally into the bloodstream.
Additional effects of IL-8 specifically related to cell restitution or angiogenesis could also be important. In other words, mucosal healing, I think, could also be affected by mediators from cells upstream.
Discussant
Dr. Carlos Chan (Montreal, Canada): I have one quick question about the slide that you showed regarding LPS treatment on the apical side. As far as I understand, the Caco-2 cells do not express Toll-like receptor 4, which is the receptor for the LPS, although you show that CD14 is expressed. So it may not respond. So have you thought of using other cell lines that you have that expresses Toll-like receptor 4?
Closing Discussant
Dr. Dennis Sonnier: Thank you for your comments and questions. Some investigators have demonstrated TLR4 expression in Caco 2 cells, at least under some conditions, but they generally are hyporesponsive to LPS due to lack of MD2 and other proteins related to LPS signaling.
Discussant
Dr. Carlos Chan (Montreal, Canada): Actually, there are some papers that show there is no receptor and some papers show there is a receptor. Have you shown on your study that these cells that you have actually have Toll-like receptor 4?
Closing Discussant
Dr. Dennis Sonnier: I have not demonstrated that myself, but unpublished data from previous residents in our lab indicate that Caco-2 cells do respond to LPS stimulation if MD2 is added to the treatments.
I would like to thank the Society for the privilege of presenting our data.
Rights and permissions
About this article
Cite this article
Sonnier, D.I., Bailey, S.R., Schuster, R.M. et al. TNF-α Induces Vectorial Secretion of IL-8 in Caco-2 Cells. J Gastrointest Surg 14, 1592–1599 (2010). https://doi.org/10.1007/s11605-010-1321-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1321-9