Abstract
Aim
The mTOR-inhibitor rapamycin has shown antitumor activity in various tumors. Bedside observations have suggested that rapamycin may be effective as a treatment for colorectal carcinomatosis.
Methods
We established an orthotopic syngenic model by transplanting CT26 peritoneal tumors in Balb/C mice and an orthotopic xenograft model by transplanting SW620 peritoneal tumors in nu/nu mice. Expression levels of tissue inhibitor of matrix-metalloproteinases 1 (TIMP-1) in the tumor and serum was determined by enzyme-linked immunosorbent assay.
Results
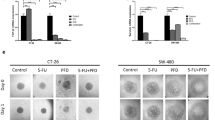
Rapamycin significantly suppressed growth of syngenic and xenografted peritoneal tumors. The effect was similar with intraperitoneal or oral rapamycin administration. Tumor suppression was further enhanced when rapamycin was combined with 5-fluorouracil and/or oxaliplatin. The combination treatment showed no acute toxicity. TIMP-1 serum levels correlated well (CC = 0.75; P < 0.01) with rapamycin treatment.
Conclusions
Rapamycin suppressed advanced stage colorectal cancer, even with oral administration. Combining rapamycin with current chemotherapy regimens significantly increased antitumor efficacy without apparent toxicity. The treatment efficacy correlated with serum TIMP-1 levels, suggesting its potential as a surrogate marker in future clinical trials.





Similar content being viewed by others
References
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5–26.
Gill S, Thomas RR, Goldberg RM. Review article: colorectal cancer chemotherapy. Aliment Pharmacol Ther 2003;18:683–692.
Chan S. Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer 2004;91:1420–1424.
Law BK. Rapamycin: an anti-cancer immunosuppressant? Crit Rev Oncol Hematol 2005;56:47–60.
Nozawa H, Watanabe T, Nagawa H. Phosphorylation of ribosomal p70 S6 kinase and rapamycin sensitivity in human colorectal cancer. Cancer Lett 2007;251:105–113.
Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 2002;8:128–135.
Guba M, Yezhelyev M, Eichhorn ME, Schmid G, Ischenko I, Papyan A, Graeb C, Seeliger H, Geissler EK, Jauch KW, Bruns CJ. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood 2005;105:4463–4469.
Seeliger H, Guba M, Koehl GE, Doenecke A, Steinbauer M, Bruns CJ, Wagner C, Frank E, Jauch KW, Geissler EK. Blockage of 2-deoxy-D-ribose-induced angiogenesis with rapamycin counteracts a thymidine phosphorylase-based escape mechanism available for colon cancer under 5-fluorouracil therapy. Clin Cancer Res 2004;10:1843–1852.
Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res 2002;62:7291–7297.
Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res 2002;62:6141–6145.
Shi Y, Frankel A, Radvanyi LG, Penn LZ, Miller RG, Mills GB. Rapamycin enhances apoptosis and increases sensitivity to cisplatin in vitro. Cancer Res 1995;55:1982–1988.
Zhang YJ, Tian XQ, Sun DF, Zhao SL, Xiong H, Fang JY. Combined inhibition of MEK and mTOR signaling inhibits initiation and progression of colorectal cancer. Cancer Invest 2009;27:273–285.
Cejka D, Preusser M, Fuereder T, Sieghart W, Werzowa J, Strommer S, Wacheck V. mTOR inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo. Anticancer Res 2008;28:3801–3808.
Leelawat K, Narong S, Udomchaiprasertkul W, Leelawat S, Tungpradubkul S. Inhibition of PI3K increases oxaliplatin sensitivity in cholangiocarcinoma cells. Cancer Cell Int 2009;9:3.
Figlin RA, Brown E, Armstrong AJ, Akerley W, Benson AB 3rd, Burstein HJ, Ettinger DS, Febbo PG, Fury MG, Hudes GR, Kies MS, Kwak EL, Morgan RJ Jr., Mortimer J, Reckamp K, Venook AP, Worden F, Yen Y. NCCN Task Force Report: mTOR inhibition in solid tumors. J Natl Compr Canc Netw 2008;6(Suppl 5):S1–S20. quiz S21-S22.
Amato RJ, Jac J, Giessinger S, Saxena S, Willis JP. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer 2009;115:2438–2446.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–2281.
Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, Jaeckle KA, Scheithauer BW, Dancey J, Hidalgo M, Walsh DJ. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 2005;23:5294–5304.
Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 2004;22:909–918.
Yukawa N, Yoshikawa T, Akaike M, Sugimasa Y, Takemiya S, Yanoma S, Imada T, Noguchi Y. Prognostic impact of tissue inhibitor of matrix metalloproteinase-1 in plasma of patients with colorectal cancer. Anticancer Res 2004;24:2101–2105.
Holten-Andersen MN, Nielsen HJ, Sorensen S, Jensen V, Brunner N, Christensen IJ. Tissue inhibitor of metalloproteinases-1 in the postoperative monitoring of colorectal cancer. Eur J Cancer 2006;42:1889–1896.
Sorensen NM, Schrohl AS, Jensen V, Christensen IJ, Nielsen HJ, Brunner N. Comparative studies of tissue inhibitor of metalloproteinases-1 in plasma, serum and tumour tissue extracts from patients with primary colorectal cancer. Scand J Gastroenterol 2008;43:186–191.
Stricklin GP, Welgus HG. Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem 1983;258:12252–12258.
Avalos BR, Kaufman SE, Tomonaga M, Williams RE, Golde DW, Gasson JC. K562 cells produce and respond to human erythroid-potentiating activity. Blood 1988;71:1720–1725.
Hewitt RE, Brown KE, Corcoran M, Stetler-Stevenson WG. Increased expression of tissue inhibitor of metalloproteinases type 1 (TIMP-1) in a more tumourigenic colon cancer cell line. J Pathol 2000;192:455–459.
Chromek M, Tullus K, Lundahl J, Brauner A. Tissue inhibitor of metalloproteinase 1 activates normal human granulocytes, protects them from apoptosis, and blocks their transmigration during inflammation. Infect Immun 2004;72:82–88.
Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 2006;25:99–113.
Luparello C, Avanzato G, Carella C, Pucci-Minafra I. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res Treat 1999;54:235–244.
Soula-Rothhut M, Coissard C, Sartelet H, Boudot C, Bellon G, Martiny L, Rothhut B. The tumor suppressor PTEN inhibits EGF-induced TSP-1 and TIMP-1 expression in FTC-133 thyroid carcinoma cells. Exp Cell Res 2005;304:187–201.
Waller JR, Brook NR, Bicknell GR, Nicholson ML. Differential effects of modern immunosuppressive agents on the development of intimal hyperplasia. Transpl Int 2004;17:9–14.
Murphy G, Knauper V, Lee MH, Amour A, Worley JR, Hutton M, Atkinson S, Rapti M, Williamson R. Role of TIMPs (tissue inhibitors of metalloproteinases) in pericellular proteolysis: the specificity is in the detail. Biochem Soc Symp 2003;70:65–80.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
Ishikawa T, Fukase Y, Yamamoto T, Sekiguchi F, Ishitsuka H. Antitumor activities of a novel fluoropyrimidine, N4-pentyloxycarbonyl-5'-deoxy-5-fluorocytidine (capecitabine). Biol Pharm Bull 1998;21(7):713–717.
Pencreach E, Guerin E, Nicolet C, Lelong-Rebel I, Voegeli AC, Oudet P, Larsen AK, Gaub MP, Guenot D. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia-inducible factor-1alpha axis. Clin Cancer Res 2009;15:1297–1307.
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–4091.
Holten-Andersen MN, Stephens RW, Nielsen HJ, Murphy G, Christensen IJ, Stetler-Stevenson W, Brunner N. High preoperative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin Cancer Res 2000;6:4292–4299.
Murphy GJ, Nicholson ML. Rapamycin has no effect on fibrosis-associated gene expression or extracellular matrix accumulation when administered to animals with established or early allograft vasculopathy. J Thorac Cardiovasc Surg 2003;126:2058–2064.
Mannello F, Gazzanelli G. Tissue inhibitors of metalloproteinases and programmed cell death: conundrums, controversies and potential implications. Apoptosis 2001;6:479–482.
Soloway PD, Alexander CM, Werb Z, Jaenisch R. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene 1996;13:2307–2314.
Acknowledgment
We would like to thank Cynthia Fuhrer (University of Bern) for her help with immunohistochemistry.
Grant support
This work was supported by the 3R Research Foundation Switzerland (no 94/04); Swiss National Foundation (SNF 3100A0-104023); Oncosuisse (OCS 01431-08-2003) (SAV).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, M., Roh, V., Strehlen, M. et al. Effective Treatment of Advanced Colorectal Cancer by Rapamycin and 5-FU/Oxaliplatin Monitored by TIMP-1. J Gastrointest Surg 13, 1781–1790 (2009). https://doi.org/10.1007/s11605-009-0948-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0948-x