Abstract
Background
Anastomotic leak related to ischemia is a source of significant morbidity and mortality in gastrointestinal surgery. The aim of this study was to apply growth factor gene transfection for the purpose of up-regulating angiogenesis, increasing anastomotic strength, and ultimately preventing dehiscence.
Methods
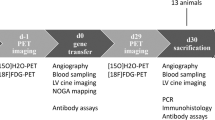
An opossum esophagogastrostomy model was employed. The human vascular endothelial growth factor (VEGF165) gene was incorporated into a recombinant plasmid. The VEGF plasmid vector was then complexed with a cationic synthetic carrier, polyethyleneimine. Control animals received plasmid devoid of VEGF165 (n = 6). The experimental group received VEGF165 plasmid (n = 5). After esophagogastrectomy and gastric tubularization, plasmid was injected into the submucosa of the neoesophagus at the anastomotic site. Conduit arteriography was performed before and 10 days after injection. Euthanasia occurred on post-injection day 10 and the anastomosis was removed en bloc. A second group of animals treated with VEGF165 were euthanized 30 and 37 days post injection. Blood flow was measured with laser-Doppler prior to euthanasia. Ex vivo anastomotic bursting pressure was performed. Tissue samples were procured for RNA extraction and von Willebrand Factor staining. Microvessel counts were obtained by two blinded observers. Tissue VEGF transcript levels were measured with reverse transcriptase polymerase chain reaction (RT-PCR).
Results
There was one anastomotic leak in the control group. Experimental animals demonstrated significantly increased bursting pressure (104.25 ± 6.2 vs 86.73 ± 9.4 mmHg, p = 0.021) and neovascularization (33.87 ± 9.6 vs 20.33 ± 8.1 vessels/hpf, p = 0.032) compared to controls. In addition, there was a strongly positive correlation between the number of microvessels and bursting pressure (r = 0.808, p = 0.015, Pearson’s). On angiographic examination, treated animals demonstrated more neovascularization compared to controls. RT-PCR demonstrated up to a 5.6-fold increase in VEGF mRNA in treated compared to controls.
Discussion
This description of gene therapy in gastrointestinal surgery using VEGF165 transfection demonstrates increased angiogenesis with subsequently improved anastomotic healing in a clinically relevant model.







Similar content being viewed by others
References
Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995;169(6):634–640. doi:10.1016/S0002-9610(99)80238-4.
Briel JW, Tamhankar AP, Hagen JA et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198(4):536–541. 541–532. discussion doi:10.1016/j.jamcollsurg.2003.11.026.
Luketich JD, Alvelo-Rivera M, Buenaventura PO et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238(4):486–494. 494–485. discussion.
Miyazaki T, Kuwano H, Kato H, Yoshikawa M, Ojima H, Tsukada K. Predictive value of blood flow in the gastric tube in anastomotic insufficiency after thoracic esophagectomy. World J Surg 2002;26(11):1319–1323. doi:10.1007/s00268-002-6366-9.
Seifert WF, Verhofstad AA, Wobbes T et al. Quantitation of angiogenesis in healing anastomoses of the rat colon. Exp Mol Pathol 1997;64(1):31–40. doi:10.1006/exmp.1997.2207.
Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992;3(2):211–220.
Brown LF, Yeo KT, Berse B et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992;176(5):1375–1379. doi:10.1084/jem.176.5.1375.
Connolly DT, Heuvelman DM, Nelson R et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–1478. doi:10.1172/JCI114322.
Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev 1992;13(1):18–32. doi:10.1210/er.13.1.18.
Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg 1999;134(2):200–205. doi:10.1001/archsurg.134.2.200.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246(4935):1306–1309. doi:10.1126/science.2479986.
Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol 1996;149(1):293–30.
Banbury J, Siemionow M, Porvasnik S, Petras S, Browne E. Improved perfusion after subcritical ischemia in muscle flaps treated with vascular endothelial growth factor. Plast Reconstr Surg 2000;106(7):1541–1546. doi:10.1097/00006534-200012000-00015.
Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ. The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg 2000;53(3):234–239. doi:10.1054/bjps.1999.3315.
Lineaweaver WC, Lei MP, Mustain W, Oswald TM, Cui D, Zhang F. Vascular endothelium growth factor, surgical delay, and skin flap survival. Ann Surg 2004;239(6):866–73. 873–865. discussion doi:10.1097/01.sla.0000128682.53915.92.
Padubidri A, Browne E Jr. Effect of vascular endothelial growth factor (VEGF) on survival of random extension of axial pattern skin flaps in the rat. Ann Plast Surg 1996;37(6):604–611. doi:10.1097/00000637-199612000-00006.
Seify H, Bilkay U, Jones G. Improvement of TRAM flap viability using human VEGF-induced angiogenesis: a comparative study of delay techniques. Plast Reconstr Surg 2003;112(4):1032–1039. doi:10.1097/01.PRS.0000076186.97093.92.
Forse RA, MacDonald PH, Mercer CD. Anastomotic and regional blood flow following esophagogastrectomy in an opossum model. J Invest Surg 1999;12(1):45–52. doi:10.1080/089419399272764.
Reavis KM, Chang EY, Hunter JG, Jobe BA. Utilization of the delay phenomenon improves blood flow and reduces collagen deposition in esophagogastric anastomoses. Ann Surg 2005;241(5):736–745. 745–737. discussion doi:10.1097/01.sla.0000160704.50657.32.
Shepherd AP, Riedel GL. Continuous measurement of intestinal mucosal blood flow by laser-Doppler velocimetry. Am J Physiol 1982;242(6):G668–G672.
Weidner N, Folkman J, Pozza F et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 1992;84(24):1875–1887. doi:10.1093/jnci/84.24.1875.
Vermeulen PB, Gasparini G, Fox SB et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 2002;38(12):1564–1579. doi:10.1016/S0959-8049(02)00094-1.
Primer3 on the WWW for general users and for biologist programmers [computer program]. Version Primer 3. Totowa, NJ: Humana Press; 2000.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29(9):45. doi:10.1093/nar/29.9.e45.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25(4):402–408. doi:10.1006/meth.2001.1262.
Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 2005;6(2):209. doi:10.1186/gb-2005-6-2-209.
Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 2000;102(8):898–901.
te Velde EA, Voest EE, van Gorp JM et al. Adverse effects of the antiangiogenic agent angiostatin on the healing of experimental colonic anastomoses. Ann Surg Oncol. 2002;9(3):303–309, Apr.
Zacchigna S, Tasciotti E, Kusmic C et al. In vivo imaging shows abnormal function of vascular endothelial growth factor-induced vasculature. Hum Gene Ther 2007;18(6):515–524. doi:10.1089/hum.2006.162.
Yamauchi A, Ito Y, Morikawa M et al. Pre-administration of angiopoietin-1 followed by VEGF induces functional and mature vascular formation in a rabbit ischemic model. J Gene Med 2003;5(11):994–1004. doi:10.1002/jgm.439.
Deng X, Szabo S, Khomenko T, Jadus MR, Yoshida M. Gene therapy with adenoviral plasmids or naked DNA of vascular endothelial growth factor and platelet-derived growth factor accelerates healing of duodenal ulcer in rats. J Pharmacol Exp Ther 2004;311(3):982–988. doi:10.1124/jpet.104.071464.
Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther 2005;12(24):1734–1751. doi:10.1038/sj.gt.3302592.
Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther 2002;9(24):1647–1652. doi:10.1038/sj.gt.3301923.
Ohlfest JR, Freese AB, Largaespada DA. Nonviral vectors for cancer gene therapy: prospects for integrating vectors and combination therapies. Curr Gene Ther 2005;5(6):629–641. doi:10.2174/156652305774964749.
Arsic N, Zentilin L, Zacchigna S et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther 2003;7(4):450–459. doi:10.1016/S1525-0016(03)00034-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
John W. Harmon, M.D. (Baltimore, MD): First, I would like to thank Dr. Enestvedt and Dr. Jobe for providing me with a copy of the manuscript. I congratulate you on nice work and a nice presentation.
As many of you in the audience are aware, the dream of gene therapy has not been fulfilled. As people say, the success of gene therapy is just around the corner, just over the horizon, and it always will be. The problem is how to deliver the gene therapy. You have viral and nonviral approaches. Dr. Enestvedt and his group are using a DNA plasmid vector which is nonviral. This avoids the problem of the systemic inflammatory response syndrome, which has notoriously appeared in certain viral trials, not often, but enough to cause a big problem. So it is very attractive that they are using a nonviral approach. So what about nonviral delivery of DNA plasmids?
The simple injection of DNA plasmidsinto tissue without anything else basically gives you a little tiny bit of transfection. We have used it, but it is not satisfactory. There is a gene gun approach in which small pellets, gold pellets, are coated with DNA, and shot into the tissue. This improves transfection efficiency, but it is highly variable and has few advocates.
Dr. Enestved's group is using a cationic lipid carrier to bring the DNA into the cells. The DNA, of course, has to get not only across the cell membrane but also across the nuclear membrane, double transport. I am impressed with this PEI transfection cationic lipid; I am impressed that it worked at all. In our hands, these cationic lipid carriers actually reduced transfection, as we have published. I looked into the literature regarding this jet PEI. It seems to be an advance, but there are conflicting reports; some people using it finding that it didn't work, others that it did.
We found that these kinds of carriers worked very well in tissue culture, especially without serum. We found that by adding serum, we progressively reduced the effectiveness of the approach, presumably as the cationic carrier with the DNA bound to the peptides, and we found they actually reduced transfection.
So, Dr. Enestvedt, my question for you is how certain are you that you are getting satisfactory transfection? You did get a nice biological response for some of your parameters. I was particularly impressed with the figure where you found a positive correlation between the bursting strength and the microvessels. However, this could possibly be a nonspecific effect.
It is of concern that real-time PCR didn't show an increase. There was an increase, but it was not statistically significant. In our lab using electroporation, instead of using the cationic lipid, we used the same kind of plasmid and the same PCR technique, we saw a 700-fold increase that lasted for a month.
Finally, I applaud you for tackling what is now becoming a classically difficult, but terribly important problem. Certainly if we could improve the healing of wounds, particularly bowel anastomoses, but many other wounds as well, using a gene therapy approach, we would be doing a very good thing for mankind, and you are part of the effort, and I applaud you.
Kristian Enestvedt, M.D. (Portland, OR): Dr. Harmon, thank you for your insightful analysis. The PCR results are definitely a concern for us. I think there are several explanations for our findings. The first is something I mentioned previously, which is the sample size. I think this does play a role. The vehicle that we chose for transfection, the PEI vehicle, I think has demonstrated a pretty reasonable effect. I think the problem with our study is the combination of the PEI with the fibrin suspension. The PEI, as you mentioned, is a strong cation, and the fibrin has a strong negative charge. This serves to tightly bind the PEI vector to the fibrin. So that when you inject it into the tissue, there is very limited lateral diffusion. If we don't sample exactly that needle tract and extract RNA from it, I think that we see less transgene in those tissue samples. The range for the fold change was on the order of 1.3 to 200. So we do see a reasonable transfection efficiency using this carrier system. I think a bigger problem is the PEI and fibrin combination sequestering the transgene in a very localized area, because, as you pointed out, we do see the appropriate downstream effect. This indicates that the VEGF growth factor is actually being disseminated to the tissue, and that is important, because that is what we need for the clinical response.
I am confident that we have addressed these issues because we have our PCR results from a second, larger study that I described, and with a doubling in the number of animals, we see a statistically significant difference in the transcript levels. In addition to the larger sample, we have removed the fibrin suspension. We are complexing only the PEI with the vector, adding PBS, injecting that solution, and now we see a significant difference in the transcript levels. Thank you, again, Dr. Harmon, for your questions.
Joerg Haier, M.D. (Muenster, Germany): First, congratulations on this very interesting approach. I have two questions, actually. Do you have an idea which type of cells is taking up your plasmid construct and which cells are actually responding to this, which means where is the VEGF coming from? And the second is, what is the advantage instead of using a slower-release, direct-protein construct?
Dr. Enestvedt: Those are excellent questions. There is a simple answer to the first:the cell types are infiltrating macrophages that are a normal response of wounding, that are abundant in the wound by postoperative day two. Those are the target cells, they take up the vectoras along with some of the native endothelial cells, and those are the cells that ultimately produce VEGF. The endothelial cells respond by sprouting new vessels.
Dr. Haier: Why are you using a genetic construct instead of direct protein release?
Dr. Enestvedt: Protein injected with a Matrigel would probably work with similar success. The problem with direct protein delivery is that the half-life is very short, and we need a sustained effect to protect the healing anastomosis. Our lab has shown in previous studies that using PEI, we have the optimal time of peak transgene expression by postoperative day four, which is when we want high VEGF levels to protect the healing anastomosis. There certainly are other methods for the delivery of VEGF but we favor this approach because of its time-course effects.
Dr. Haier: But there are some glycocalix coatings for suture materials available that release the proteins in similar kinetics. It might be an option too.
Dr. Enestvedt: Thank you, that is an excellent suggestion.
This manuscript was presented at the Society for Surgery of the Alimentary Tract/Digestive Disease Week meeting in San Diego, CA, May 2008.
Research Support: This work was supported in part by National Institutes of Health grants K-23 DK066165 (BAJ) and K-08 DK074397 (RWO), the Frank W. Jobe Foundation (BAJ, CKE), an American Surgical Association Career Development Award (RWO), and a Medical Research Foundation of Oregon Early Clinical Investigator Award (CKE).
Rights and permissions
About this article
Cite this article
Enestvedt, C.K., Hosack, L., Winn, S.R. et al. VEGF Gene Therapy Augments Localized Angiogenesis and Promotes Anastomotic Wound Healing: A Pilot Study in a Clinically Relevant Animal Model. J Gastrointest Surg 12, 1762–1772 (2008). https://doi.org/10.1007/s11605-008-0635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0635-3