Abstract
Background
American Joint Committee on Cancer (AJCC) staging for pancreatic adenocarcinoma is a validated predictor of prognosis but insufficiently discriminates postresection survival. We hypothesized that genetic analysis of resected cancers would correlate with tumor biology and postoperative survival.
Methods
Resected pancreatic ductal and ampullary adenocarcinomas (n = 50) were analyzed for loss of heterozygosity (LOH) at 15 markers including 5q(APC), 6q(TBSP2), 9p(p16), 10q(PTEN), 12q(MDM2), 17p(TP53), and 18q(DCC/SMAD4). KRAS exon 1 mutations were detected by sequencing. The primary endpoint of this interim data analysis was survival at 18 month median follow-up.
Results
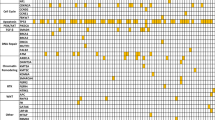
Negative margins were achieved in 43 (86%) cases. AJCC stage was: Ia/b (3), IIa (16), IIb (31). KRAS mutations were detected in 31 cases (62%) and LOH in 26 (52%) with mean fractional allelic loss score 23 ± 16%. Median survival was significantly shorter with LOH (15.2 months versus not reached; p = 0.021) and KRAS mutations (19.6 months versus not reached; p = 0.038). Combining KRAS mutation with LOH was a powerful negative predictor in Cox regression (HR = 10.6, p = 0.006). Stage, nodal and margin status were not predictive of survival.
Conclusion
LOH and KRAS mutations indicate aggressive tumor biology and correlate strongly with survival in resected pancreatic ductal and ampullary carcinomas. Genetic analysis may improve risk stratification in future clinical trials.


Similar content being viewed by others
References
Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 1999;189:1–7. doi:10.1016/S1072-7515(99)00075-7.
Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173–180. doi:10.1097/SLA.0b013e3180691579.
Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 2003;138:427–423. discussion 33–4. doi:10.1001/archsurg.138.4.427.
Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10–15. doi:10.1097/01.sla.0000217673.04165.ea.
Makary MA, Winter JM, Cameron JL, Campbell KA, Chang D, Cunningham SC et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg 2006;10:347–356. doi:10.1016/j.gassur.2005.12.014.
de Castro SM, Kuhlmann KF, van Heek NT, Busch OR, Offerhaus GJ, van Gulik TM et al. Recurrent disease after microscopically radical (R0) resection of periampullary adenocarcinoma in patients without adjuvant therapy. J Gastrointest Surg 2004;8:775–784. discussion 84. doi:10.1016/j.gassur.2004.08.006.
Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 1997;21:195–200. doi:10.1007/s002689900215.
Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg 2008;12:701–706. doi:10.1007/s11605-007-0384-8.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236:355–366. discussion 66–8. doi:10.1097/00000658-200209000-00012.
Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery 1973;73:307–320.
Fortner JG, Klimstra DS, Senie RT, Maclean BJ. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg 1996;223:147–153. doi:10.1097/00000658-199602000-00006.
Greene F, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al, editors. AJCC Cancer Staging Manual. 6 ed: Springer; 2002.
Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg 2004;240:293–298. doi:10.1097/01.sla.0000133125.85489.07.
Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610–618. doi:10.1016/j.surg.2006.12.013.
Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP et al. Validation of the 6(th) edition AJCC pancreatic cancer staging system: report from the National Cancer Database. Cancer 2007;110:738–744. doi:10.1002/cncr.22852.
Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol 2005;23:7529–7535. doi:10.1200/JCO.2005.01.8101.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–2826. doi:10.1056/NEJMoa041588.
Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25. doi:10.1186/bcr1412.
Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 2007;25:587–595. doi:10.1200/JCO.2006.07.3585.
Mina L, Soule SE, Badve S, Baehner FL, Baker J, Cronin M et al. Predicting response to primary chemotherapy: gene expression profiling of paraffin-embedded core biopsy tissue. Breast Cancer Res Treat 2007;103:197–208. doi:10.1007/s10549-006-9366-x.
Marchionni L, Wilson RF, Wolff AC, Marinopoulos S, Parmigiani G, Bass EB et al. Systematic review: gene expression profiling assays in early-stage breast cancer. Ann Intern Med 2008; 148:358–369.
Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 2001;344:1196–1206. doi:10.1056/NEJM200104193441603.
Kojima K, Vickers SM, Adsay NV, Jhala NC, Kim HG, Schoeb TR et al. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res. 2007;67:8121–8130. doi:10.1158/0008-5472.CAN-06-4167.
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988;53:549–554. doi:10.1016/0092-8674(88)90571-5.
Fung YK, Murphree AL, T’Ang A, Qian J, Hinrichs SH, Benedict WF. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987;236:1657–1661. doi:10.1126/science.2885916.
Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res 2000;6:2969–2972.
Kern SE, Hruban RH, Hidalgo M, Yeo CJ. An introduction to pancreatic adenocarcinoma genetics, pathology and therapy. Cancer Biol Ther 2002;1:607–613.
Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA 2000;97:9624–9629. doi:10.1073/pnas.97.17.9624.
Moore PS, Beghelli S, Zamboni G, Scarpa A. Genetic abnormalities in pancreatic cancer. Mol Cancer 2003;2:7. doi:10.1186/1476-4598-2-7.
Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas 1996;12:373–380. doi:10.1097/00006676-199605000-00009.
Marsh JW, Finkelstein SD, Demetris AJ, Swalsky PA, Sasatomi E, Bandos A et al. Genotyping of hepatocellular carcinoma in liver transplant recipients adds predictive power for determining recurrence-free survival. Liver Transplant 2003;9:664–671. doi:10.1053/jlts.2003.50144.
Momand J, Wu HH, Dasgupta G. MDM2–master regulator of the p53 tumor suppressor protein. Gene 2000;242:15–29. doi:10.1016/S0378-1119(99)00487-4.
Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83. discussion. doi:10.1001/archsurg.2007.17.
Stocken DD, Buchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92:1372–1381. doi:10.1038/sj.bjc.6602513.
Yatsuoka T, Sunamura M, Furukawa T, Fukushige S, Yokoyama T, Inoue H et al. Association of poor prognosis with loss of 12q, 17p, and 18q, and concordant loss of 6q/17p and 12q/18q in human pancreatic ductal adenocarcinoma. Am J Gastroenterol 2000;95:2080–2085. doi:10.1111/j.1572-0241.2000.02171.x.
Mahlamaki EH, Hoglund M, Gorunova L, Karhu R, Dawiskiba S, Andren-Sandberg A et al. Comparative genomic hybridization reveals frequent gains of 20q, 8q, 11q, 12p, and 17q, and losses of 18q, 9p, and 15q in pancreatic cancer. Genes Chromosomes Cancer 1997; 20:383–391 doi:10.1002/(SICI)1098-2264(199712)20:4<383::AID-GCC10>3.0.CO;2-O.
Iacobuzio-Donahue CA, van der Heijden MS, Baumgartner MR, Troup WJ, Romm JM, Doheny K et al. Large-scale allelotype of pancreaticobiliary carcinoma provides quantitative estimates of genome-wide allelic loss. Cancer Res 2004;64:871–875. doi:10.1158/0008-5472.CAN-03-2756.
Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M et al. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol 2007;13:3714–3720.
Yamano M, Fujii H, Takagaki T, Kadowaki N, Watanabe H, Shirai T. Genetic progression and divergence in pancreatic carcinoma. Am J Pathol 2000;156:2123–2133.
Giroux V, Malicet C, Barthet M, Gironella M, Archange C, Dagorn JC et al. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin Cancer Res 2006;12:235–241. doi:10.1158/1078-0432.CCR-05-1700.
Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res 2002;62:2890–2896.
Iacobuzio-Donahue CA, Maitra A, Olsen M, Lowe AW, van Heek NT, Rosty C et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol 2003;162:1151–1162.
Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS et al. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res 1995;55:4670–4675.
Lefter LP, Sunamura M, Furukawa T, Takeda K, Kotobuki N, Oshimura M et al. Inserting chromosome 18 into pancreatic cancer cells switches them to a dormant metastatic phenotype. Clin Cancer Res 2003;9:5044–5052.
Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science 1998;280:1036–1037. doi:10.1126/science.280.5366.1036.
Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P et al. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA 1999;96:14888–14893. doi:10.1073/pnas.96.26.14888.
Hirama T, Koeffler HP. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood 1995;86:841–854.
Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol 2005;11:2162–2165.
Bollag G, McCormick F. Regulators and effectors of ras proteins. Annu Rev Cell Biol 1991;7:601–632. doi:10.1146/annurev.cb.07.110191.003125.
Ikeda N, Nakajima Y, Sho M, Adachi M, Huang CL, Iki K et al. The association of K-ras gene mutation and vascular endothelial growth factor gene expression in pancreatic carcinoma. Cancer 2001;92:488–499. doi:10.1002/1097-0142(20010801)92:3<488::AID-CNCR1347>3.0.CO;2-F.
Berger DH, Chang H, Wood M, Huang L, Heath CW, Lehman T et al. Mutational activation of K-ras in nonneoplastic exocrine pancreatic lesions in relation to cigarette smoking status. Cancer 1999;85:326–332. doi:10.1002/(SICI)1097-0142(19990115)85:2<326::AID-CNCR9>3.0.CO;2-O.
Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 1998;10:262–267. doi:10.1016/S0955-0674(98)80149-X.
Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935–949. discussion 49–50. doi:10.1016/j.gassur.2004.09.046.
Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol 2006;13:1189–1200. doi:10.1245/s10434-006-9016-x.
Strom A, Bonal C, Ashery-Padan R, Hashimoto N, Campos ML, Trumpp A et al. Unique mechanisms of growth regulation and tumor suppression upon Apc inactivation in the pancreas. Development 2007;134:2719–2725. doi:10.1242/dev.02875.
Schniewind B, Groth S, Sebens Muerkoster S, Sipos B, Schafer H, Kalthoff H et al. Dissecting the role of TGF-beta type I receptor/ALK5 in pancreatic ductal adenocarcinoma: Smad activation is crucial for both the tumor suppressive and prometastatic function. Oncogene 2007;26:4850–4862. doi:10.1038/sj.onc.1210272.
Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 1997;57:3126–3130.
Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA 2008;105:3933–3938. doi:10.1073/pnas.0708917105.
Carroll VA, Ashcroft M. Regulation of angiogenic factors by HDM2 in renal cell carcinoma. Cancer Res 2008;68:545–552. doi:10.1158/0008-5472.CAN-06-4738.
Okamura S, Arakawa H, Tanaka T, Nakanishi H, Ng CC, Taya Y et al. p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis. Mol Cell 2001;8:85–94. doi:10.1016/S1097-2765(01)00284-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
Mark P. Callery, M.D. (Boston, MA): Dr. Franko, my congratulations to you and Dr. Jim Moser and your Pittsburgh colleagues on this excellent plenary paper. Custom tumor genotypic profiling will be, for sure, one wave of the future as we migrate to custom tumor therapies based on actual tumor biology.
My two questions are, and you actually stressed the first one a bit with your combined defects, how does your data support the concept that tumor biology is worse when multiple cumulative genetic defects emerge? And my second question: Were you able to reconcile and segregate using your microdissection technique relative genetic defects between ductal cells and surrounding stromal cells? This could be a novel and worthwhile tactic for your research.
Congratulations.
Jan Franko, M.D., Ph.D. (Pittsburgh, PA): Thank you, Dr. Callery, for excellent questions. I will answer the second question first. I think it is very important to deal with the stroma. We did not do that in this study. I think it is a great idea for our future plans. And it is especially true when we look at the recent presentation on Sunday from the Pancreas Club where one of the groups presented the epithelial-to-mesenchymal transition results, and if that would be true, actually the whole issue regarding analysis of surgical margin comes into question. If one doesn’t see malignant ductal cells at the margin, we call this margin histologically negative, when in fact there may be mesenchymal-like cells which represent cancer. So I think LOH analysis or any genetic technique to be used for the stroma is important, and I think it is something that we will get into, and hopefully we will be able to report soon.
If I am correct, the first question was related to differences between –
Dr. Callery: I wrote my question before I heard your updated slide. You answered it. The accumulated combined KRAS-LOH defects that support a multi-hit theory.
O. Joe Hines, M.D. (Los Angeles, CA): I like the concept, it is a great concept, and obviously it is the direction we are going to be going, but I want to ask you a specific question as to how you did the study. It seems that ampullary and pancreatic cancers were pooled in your analysis. I think that these are really two very different diseases. I know for many, many years pancreatic surgeons have reported their clinical data pooled and called this periampullary disease. Have you looked to separate the two groups, and what do you think about this issue of pooling ampullary and pancreatic together?
Dr. Franko: I think it is right to the point. Thank you for that question. Everything is relative. Many say that ampullary cancer has a substantially better prognosis, and it is mostly true because most patients are caught in early stage of disease. But when you go to stage by stage, even in a plenary session a couple of speeches before me, it was demonstrated they have pretty poor survival. I am not saying they are exactly the same. But if you go stage by stage, the survival is not that different.
At the time when I constructed the study, it came to the question of power to collect enough data, enough patients; I think only seven patients, about 14%, represent ampullary origin. If I do a sensitivity and subgroup analysis with the current follow-up as of today, all what I have said here holds true for pancreatic ductal carcinoma. It doesn’t make too much sense to examine seven patients with ampullary carcinoma separatelly. In the future we will collect over 180 specimens, which is our current plan. We will separate pancreatic ductal and ampullary histologies.
I allowed to pool those together, because there is a nice review from Dr. Moore from I think 2004 where he described the current evidence and suggested that the genetic abnormalities in ampullary and pancreatic ductal actually are very similar. So that was the mechanistic reason why I pooled them together, and also I needed it for power of the study.
Andreas C. Hoffmann, M.D. (Los Angeles, CA): How did you do the PCR analysis? You said that you did PCR. I myself tried the k-ras as well. Did you use a probe method, such that you changed the probe in the design to detect the mutation, and did you correlate your PCR analysis with gene sequencing data?
Dr. Franko: The KRAS was done by gene sequencing. The LOH analysis is not necessarily done by PCR. It is amplified by PCR. But what we actually measure is the fluorescence of the markers which are tagged, and you compare normal tissue versus the malignant epithelium. So PRC is used just to get more signal, but it is not true PCR for LOH analysis, as opposed to KRAS. That is done through sequencing.
Support: Koch Regional Perfusion Center and the John F. Fortney Pancreatic Cancer Research Foundation
Rights and permissions
About this article
Cite this article
Franko, J., Krasinskas, A.M., Nikiforova, M.N. et al. Loss of Heterozygosity Predicts Poor Survival After Resection of Pancreatic Adenocarcinoma. J Gastrointest Surg 12, 1664–1673 (2008). https://doi.org/10.1007/s11605-008-0577-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0577-9