Abstract
The natural history of pancreatic neuroendocrine tumors (PNET) remains poorly defined. Our objectives were to examine the clinicopathologic features of PNETs, to assess treatment trends over time, and to identify factors associated with undergoing resection. From the National Cancer Data Base (1985–2004), 9,821 patients were identified with PNETs. Clinicopathologic features and treatment trends were examined. Multivariable logistic regression was used to assess factors associated with undergoing resection. Of 9,821 patients with PNETs, 85% were nonfunctional, 7.1% were functional, and 7.9% were carcinoid tumors. Of the 3,851 (39.0%) patients who underwent pancreatectomy, 449 (11.7%) received adjuvant chemotherapy, and 254 (6.6%) received adjuvant radiation. From 1985 to 2004, utilization of pancreatectomy increased from 39.4 to 44.3% (P < 0.0001). Patients were less likely to undergo resection if they were >55 years old, had tumors in the head of the pancreas, tumors ≥4 cm, or had distant metastases (P < 0.0001). Patients treated at NCCN/NCI, academic, or high-volume hospitals were more likely to undergo resection. There are disparities in the utilization of pancreatectomy for PNETs. As PNETs have a better prognosis than adenocarcinoma, concerns regarding the morbidity and mortality of pancreatic surgery and neoplasms should not preclude resection.


Similar content being viewed by others
References
Mullan MH, Gauger PG, Thompson NW. Endocrine tumours of the pancreas: review and recent advances. ANZ J Surg 2001;71(8):475–482.
Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2005;14(7):1766–1773.
Fitzgerald T, Hickner Z, Schmitz M, et al. Increasing incidence of nonfunctional neuroendocrine tumors of the pancreas. American Society of Clinical Oncology Gastrointestinal Cancers Symposium. Orlando, FL., 2007.
Capella C, Heitz PU, Hofler H, et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch 1995;425(6):547–560.
Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995;130(3):295–299 (discussion 299–300).
Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg 2003;27(3):324–329.
Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004;139(7):718–725 (discussion 725–727).
Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4(6):567–579.
Whipple AO. An evaluation of radical surgery for carcinoma of the pancreas and ampullary region. Ann Intern Med 1949;31(4):624–627.
Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244(1):10–15.
Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 1995;222(5):638–645.
Jarufe NP, Coldham C, Orug T, et al. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg 2005;22(3):157–162.
Norton JA. Surgery for primary pancreatic neuroendocrine tumors. J Gastrointest Surg 2006;10(3):327–331.
Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol 2004;85(1):1–3.
Guo KJ, Liao HH, Tian YL, et al. Surgical treatment of nonfunctioning islet cell tumor: report of 41 cases. Hepatobiliary Pancreat Dis Int 2004;3(3):469–472.
Commission on Cancer. Standards of the Commission on Cancer Volume II: Registry operations and data standards. Chicago: American College of Surgeons, 1998.
Facility Oncology Registry Data Standards. Chicago: American College of Surgeons, Commission on Cancer, 2004.
Commission on Cancer: Approvals Categories. http://www.facs.org/cancer/coc/categories.html, 2006.
Hosmer J, Lemeshow S. Applied Logistic Regression. New York: Wiley, 1999.
United States Census Bureau. Census 2000. http://www.census.gov/main/www/cen2000.html. Accessed January 21, 2007.
Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol 2002;20(11):2633–2642.
Madeira I, Terris B, Voss M, et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut 1998;43(3):422–427.
Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12(4):1083–1092.
Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg 2007;245(2):273–281.
National Comprehensive Cancer Network (NCCN): Neuroendocrine Tumors. http://www.nccn.org, 2007.
Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 2005;241(5):776–783 (discussion 783–785).
Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190(4):432–445.
Bilimoria K, Bentrem D, Ko C, et al. National failure to operate on early-stage pancreatic cancer. Ann Surg 2007 (On line).
O’Connell JB, Maggard MA, Ko CY. Cancer-directed surgery for localized disease: decreased use in the elderly. Ann Surg Oncol 2004;11(11):962–969.
Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg 2006;10(3):347–356.
Bilimoria K, Bentrem D, Ko C, et al. National failure to operate on early-stage pancreatic cancer in the United States. 2007 (in press).
Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280(20):1747–1751.
Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993;217(5):430–435 (discussion 435–438).
Chung JC, Choi DW, Jo SH, et al. Malignant nonfunctioning endocrine tumors of the pancreas: Predictive factors for survival after surgical Treatment. World J Surg 2007.
Liang H, Wang P, Wang XN, et al. Management of nonfunctioning islet cell tumors. World J Gastroenterol 2004;10(12):1806–1809.
Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery 2001;130(6):1078–1085.
Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 2005;16(11):1806–1810.
Venkatesh S, Ordonez NG, Ajani J, et al. Islet cell carcinoma of the pancreas. A study of 98 patients. Cancer 1990;65(2):354–357.
Acknowledgements
KYB is supported by the American College of Surgeons, Clinical Scholars in Residence program and a research grant from the Department of Surgery, Feinberg School of Medicine, Northwestern University. This study was presented in part at the 48th Annual Meeting of the Society for Surgery of the Alimentary Tract (SSAT) meeting at Digestive Disease Week 2007 in Washington, DC on May 23, 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
C. Max Schmidt, M.D. (Indianapolis, IN): Congratulations, Karl, on an excellent study. I have enjoyed watching you continue to succeed as a clinician scientist.
Dr. Bilimoria and his group presented the largest study that I know of pancreatic neuroendocrine tumors, defining their clinical and pathological characteristics and outcomes, finding that age, grade, as well as distant metastases, and not size predict survival after resection. I have a few questions for you and your co-authors.
Pancreatic neuroendocrine tumors, as you noted in your talk and in your paper, in the National Cancer Center Data Base are included only when they are determined to be malignant as opposed to benign by the pathologist. This is rather intriguing because I am not sure how this would be determined. Did you talk to any pathologists at your institution or others to try to get an idea of maybe the proportion of tumors that have been categorized as benign and what characteristics they might have?
Secondly, you have done extensive analysis with this data, and you have looked at factors that predict whether or not patients with pancreatic neuroendocrine tumors will undergo surgical resection. Interestingly, size and location are predictive factors, i.e., larger size and location in the head of the pancreas, which are not associated with resection. In the last 20 years, despite the fact that surgical resection is the only effective treatment, we have only increased the number of resections for this cancer by 5%. So my question to you is, how are we going to get this message to the community? Fifty percent of these tumors are resected in academic centers or National Cancer Institute centers, and 50% are resected in the community.
The third question is about your prognostic score in resected patients. Interestingly, age, grade and distant metastases were utilized in your score and all significant on multivariate analysis. I would like you to speculate why age in resected patients is associated with survival? Eventually all of us must die, but there have been some nice studies to suggest that pancreatic surgery in the elderly is safe, and perhaps you can comment if it is different in terms of age predicting survival in the community versus the academic setting?
And finally, you mentioned some ways in which you are going to validate your prognostic score, but I have not worked with the National Cancer Data Base. I wonder if there is a way to prospectively use the database to validate from here onwards?
Thank you for the privilege of the floor.
Dr. Bilimoria: Thank you for those insightful questions. First, it was a source of irritation when realized that only the malignant tumors are reported to cancer registries. This is partly due to the WHO classification of 2000 where they try to make the distinction between benign and malignant, and our pathologists give this no credence and don't understand the WHO’s distinction between benign and malignant. They don't think it is a useful system. Other people have classified benign versus malignant in the literature by patients who have metastases vs. no metastases or nodal involvement vs. no nodal involvement to classify the tumor as benign or malignant, and so it is sort of a confounded definition. I think that it is very unclear, and I think it shouldn't be used, and I hope that we can have all of these tumors reported to the National Cancer Data Base regardless of whether they are deemed benign or malignant.
As far as utilization goes, it is similar to our work with pancreatic adenocarcinoma where we found that nearly 40% of patients were not undergoing surgery for resectable disease. We saw the same thing here, and age, size and tumor location really affected utilization of surgery. Certainly the issue of location of the tumor is probably based on historical concerns with the Whipple procedure, such as high perioperative mortality and complications. Thus, those views are based on outdated data. It probably goes to the point of trying to influence our community hospitals and surrounding hospitals to utilize surgery for this disease.
And that goes to the next question of how are we going to get the message out. It is not the people in this room who won't operate on pancreatic cancer. So it is the people in this room who can spread the message and take it to other surrounding hospitals and state medical societies and continue to educate and update the medical community on the improving data for pancreatic cancer resection.
Your third question was regarding the importance of age on utilization of surgery. A study by Dr. Makary and colleagues from Hopkins looked at age for the Whipple procedure and found that old patients did pretty well, and I think Dr. Cameron's review of 1,000 Whipples actually had a 103-year-old patient who underwent a Whipple procedure. I agree that we need to think about pushing the limit, but that may speak directly to what we were talking about next door yesterday. It’s the system and the team that are really involved in being able to take care of the elderly patient. It is the ICU care, the anesthesia care, and so forth. If that turns out to be the case, then maybe referral for those older patients is important. We are going to submit some data to that effect looking at 15 different cancers very soon. Also age is an important prognostic factor no matter what cancer you look at. In a multivariate model, age was the most powerful predictor of outcome. Simply having age in the model doesn't completely account for it, but it does to some extent, and it is a powerful predictor of survival. Older people die, old people have more comorbidities, and so it is also a proxy for the severity of comorbidities. There were no differences between academic and community hospitals in the multivariate models by age, but community hospitals on univariate analysis typically, as we saw in the rectal cancer paper, have older patients and actually they have sicker patients than at the academic hospitals by Charlson score.
Finally, to validate the model using a prospective system is exactly something that we would like to look into. SEER in the Detroit and LA regions allow investigators to get the names of patients and follow them once they have been diagnosed, and this happens relatively quickly after diagnosis so you can actually do a study early on. The National Cancer Data Base isn't set up that way and there are some limitations because we have so much sensitive data on the hospital and the patient that we cannot to give out freely at this point. We are looking at developing a public use data set. But to do this prospectively where we can identify patients quickly after diagnosis and include them in clinical trials would be fantastic, and I think that we need to take a lesson from SEER on how to do that and move in that direction. That is a great idea.
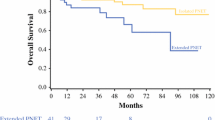
Dr. K. Lillemoe (Indianapolis, IN): Karl, another nice bit of work from you and your group. I do quibble a little bit with this study, though, because I really think you are mixing apples with oranges. All these tumors are rare, but I think clinicians have got an idea of the natural history of gastrinomas, they have an idea of the natural history of carcinoids, insulomas, as we have talked before, the rare one that is malignant, falls into your group. The big unknown is the nonfunctional neuroendocrine or islet cell tumors which make up the vast majority of your cases. So I guess my question for you is, if you just threw out all the functional tumors and just focused on the nonfunctional islet tumors, is this a valid tool, because that is where we need help in telling patients what to do. Obviously we don't have a lot of options in terms of adjuvant therapies, but clearly those are the big unknown and that is where I really question if you could mine your database to answer the real prognosis with those tumors.
Another nice bit of work from your group.
Dr. Bilimoria: We did exactly that. We excluded the carcinoid tumors and the functional tumors separately and then we excluded both groups, the carcinoid and the functional tumors. When we just looked at the nonfunctional tumors, the remaining 83% of tumors that underwent resection, the prognostic score held up in exactly the same way. The magnitude and direction of the hazard ratios in the Cox model were almost identical to when we included the functional and/or carcinoid tumors.
Dr. Lillemoe: And lymph nodes didn't have any prognosis with the nonfunctional?
Dr. Bilimoria: No, they did not.
Dr. H. Chen (Madison, WI): Fantastic study. Congratulations. I just had a quick question for clarification. In your carcinoids, were all those pancreatic carcinoids or did you throw in gastrointestinal carcinoids in your study?
Dr. Bilimoria: They were purely pancreatic carcinoids. These did not include peripancreatic or other gastrointestinal tumors. They had to be coded as a primary pancreatic neoplasm to be included in our study.
Dr. B. Clary (Durham, NC): One last question. For your multivariate analysis, that was done on the entire population. If you limited it to your resected patients, do nodal status and margin become important and should you create your prognostic scoring system from that population?
Dr. Bilimoria: Sorry if I didn't make that clear. The score and the prognostic factors are based entirely on resected patients. We did the analyses on all patients as well, but what I have shown you here is only on the resected patients.
Rights and permissions
About this article
Cite this article
Bilimoria, K.Y., Tomlinson, J.S., Merkow, R.P. et al. Clinicopathologic Features and Treatment Trends of Pancreatic Neuroendocrine Tumors: Analysis of 9,821 Patients. J Gastrointest Surg 11, 1460–1469 (2007). https://doi.org/10.1007/s11605-007-0263-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0263-3