Abstract
Introduction
Obese individuals may have normal insulin–glucose homeostasis, insulin resistance, or diabetes mellitus. Whereas gastric bypass cures insulin resistance and diabetes mellitus, its effects on normal physiology have not been described. We studied insulin resistance and β-cell function for patients undergoing gastric bypass.
Methods
One hundred thirty-eight patients undergoing gastric bypass had fasting insulin and glucose levels drawn on days 0, 12, 40, 180, and 365. Thirty-one (22%) patients with diabetes mellitus were excluded from this analysis. Homeostatic model of assessment was used to estimate insulin resistance, insulin sensitivity, and β-cell function. Based on this model, patients were categorized as high insulin resistance if their insulin resistance was >2.3.
Results
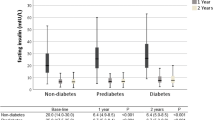
Body mass index did not correlate with insulin resistance. Forty-seven (34%) patients were categorized as high insulin resistance. Correction of insulin resistance for this group occurred by 12 days postoperatively. Sixty (43%) patients were categorized as low insulin resistance. They demonstrated an increase of β-cell function by 12 days postoperatively, which returned to baseline by 6 months. At 1 year postoperatively, the low insulin resistance group had significantly higher β-cell function per degree of insulin sensitivity.
Conclusions
Adipose mass alone cannot explain insulin resistance. Severely obese individuals can be categorized by degree of insulin resistance, and the effect of gastric bypass depends upon this preoperative physiology.





Similar content being viewed by others
References
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282(16):1523–1529.
Allison DB, Fontaine KR, Manson JE, Stevens J, van Itallie TB. Annual deaths attributable to obesity in the United States. JAMA 1999;282(16):1530–1538.
Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992;41:715–722.
Kuusisto J, Lempiainen P, Mykkänen L, Laakso M. Insulin resistance syndrome predicts coronary heart disease events in elderly type 2 diabetic men. Diabetes Care 2001;24(9):1629–1633.
McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the Atherosclerosis Risk in Communities Study. Diabetes Care 2005;28(2):385–390.
Pories WJ, Swanson MS, MacDonald KG Jr. et al. Who would have thought it? An operation proves to be the most effective therapy for diabetes mellitus. Ann Surg 1995;222(3):339–352.
MacDonald KG Jr, Long SD, Swanson MS et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997;1(3):213–220.
Sirinek KR, O’Dorisio TM, Hill D, McFee AS. Hyperinsulinism, glucose-dependent insulinotropic polypeptide, and the enteroinsular axis in morbidly obese patients before and after gastric bypass. Surgery 1986;100:781–787.
Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 1990;211:763–771.
Hickey MS, Pories WJ, MacDonald KG, Cory KA, Dohm GL, Swanson MS, Israel RG, Barakat HA, Considine RV, Caro JF, Houmard JA. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg 1998;227:637–644.
Pereira JA, Lazarin MACT, Pareja JC, de Souza A, Muscelli E. Insulin resistance in nondiabetic morbidly obese patients: effects of bariatric surgery. Obes Res 2003;11:1495–1501.
Lee WJ, Huang MT, Wang W, Lin C, Chen T, Lai I. Effects of obesity surgery on the metabolic syndrome. Arch Surg 2004;139:1088–1092.
Vague J. The degree of masculine differentiation of obesities: a factor for determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956;4:20–33.
Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005;353(3):249–254.
Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects; evidence for a hyperbolic function. Diabetes 1993;42:1663–1672.
Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess β-cell function in clinical studies. Eur J Endocrinol 2004;150:97–104.
Consensus Development Conference Panel, National Institutes of Health. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991;115:956–961.
Kadish AH, Little RL, Sternberg JC. A new and rapid method for the determination of glucose by measurement of rate oxygen consumption. Clin Chem 1968;144:116.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and â-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419.
Levy J, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21(12):2191–2192.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27(6):1487–1495.
Laird NM, Donnelly C, Ware JH. Longitudinal studies with continuous responses. Stat Methods Med Res 1992;1:3–25.
Littell RC, Milliken GA, Stroup WW et al. The SAS System for Mixed Models. Cary, NC: SAS Institute Inc., 1996.
McLean RA, Sanders WL, Stroup WW. A unified approach to mixed linear models. Am Stat 1991;45:54–64.
Corbeil RR, Searle SR. Restricted maximum likelihood (REML) estimation of variance components in the mixed model. Technometrics 1976;18:31–38.
Morrell CH. Likelihood ratio testing of variance components in the linear mixed-effects model using restricted maximum likelihood. Biometrics 1998;54:1560–1568.
Laird NM, Lange N, Stram DO. Maximum likelihood computations with repeated measures: application of the EM Algorithm. J Am Stat Assoc 1987;82:97–105.
Mikulich SK, Zerbe GO, Jones RH et al. Relating the classical covariance adjustment techniques of multivariate growth curve models to modern univariate mixed effects models. Biometrics 1999;55:957–964.
SPSS Inc. SPSS Version 12.0 for Windows. Chicago, IL: SPSS Inc., 2003.
SAS Institute Inc. SAS 9.1.3. Cary, NC: SAS Institute Inc., 2006.
Littell R, Wolfinger R. Mixed model analysis of data with the SAS system. Orlando, FL: Walt Disney World Dolphin–Salon V, 1995, pp 1–93.
SAS Institute Inc. The MIXED procedure. In: SAS/STAT Software: Changes and Enhancements Through Release 6.12. Cary, NC: SAS Institute, Inc., 1997, pp 571–702.
Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of insulin resistance using routine clinical measurements. Diabetes 2005;54:333–339.
Bonora E, Saggiani F, Targher G, Zenere MB, Aiberiche M, Monauni T, Bonadonna RC, Muggeo M. Homeostasis model Assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23(1):57–62.
Flum DR, Salem L, Elrod JB, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA 2005;294(15):1903–1908.
Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA 2005;294(15):1909–1917.
Weiss R, Dziura J, Burget TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–2374.
St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans. Diabetes Care 2004;27(9):2222–2228.
Savage DB, Tan GD, Acerini CL, Jeb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD, Dixon AK, Dunne F, Boiani R, Cinit S, Vidal-Puig A, Karpe F, Chatterjee VKK, O’Rahilly S. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferators-activated receptor-c. Diabetes 2003;52:910–917.
Al-Shali K, Cao H, Knoers N, Hermus AR, Tack CJ, Hegele RA. A single base mutation in the peroxisome proliferators-activated receptor γ4 promoter associated with altered in vitro expression and partial lipodystrophy. J Clin Endocrinol Metab 2004;89:5655–5660.
Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol 2004;150:97–104.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCUSSION
Dr. M. Sarr (Rochester, MN): Dr. Perugini and I have discussed this paper beforehand, and I will have to admit I have had a very, very difficult time understanding several of the concepts of this work.
First, I will remind the audience, these are nondiabetics, some of whom have insulin resistance and some of whom might be considered as having the metabolic X syndrome even though they are not hyperglycemic. That is a new concept for many of us.
Second, the derivation of the HOMA-IR score—that is, the score that defines insulin resistance—is not well-defined. Rich, I think you should tell us how that was done, because you take the fasting insulin concentration in the blood – and, again, these are fasting studies – and the blood glucose concentration and from those two parameters estimate the insulin resistance.
Third, the hypothesis being proposed is that once the nondiabetics that do not have insulin resistance lose weight, they might be the group that has a higher relative amount of insulin secreted than normal for a certain fasting blood glucose, and they may actually be the patients who develop noninsulinoma hyperinsulinemic hypoglycemia postoperatively.
There are, however, several assumptions. One is that postprandial insulin metabolism and homeostasis is the same as what you are estimating from your fasting studies. Maybe you could discuss this point.
Second, just out of interest, were you able to correlate body fat distribution in these nondiabetics with their insulin resistance, according to whether they had central obesity or peripheral obesity?
Dr. Perugini: The homeostasis model of assessment is a computer model generated at Oxford University and first published in the mid-‘80s. The problem is that the gold standard for studying insulin sensitivity and beta cell function is with the insulin and glucose clamp. This particular procedure requires a patient to be on bedrest for up to seven hours and requires two peripheral lines to be started, one of which infuses glucose, and one of which infuses insulin. It is not useful in the clinical setting because it is so cumbersome. Researchers at Oxford performed insulin and glucose clamps on a large body of patients; they then developed a computer program to model the outcomes based on the fasting insulin and the fasting glucose level.
Now, there are a bunch of theoretical presumptions that they made. Suffice it to say, when you compare the results from the HOMA to the results from an insulin and glucose clamp, the correlation coefficient is actually very high; the R is about .7.
Whenever big, broad-based epidemiologic studies examine whether insulin resistance has any impact on cardiovascular morbidity, nobody can use a clamp, for the same pragmatic reasons I just mentioned. These studies typically use calculations to estimate insulin sensitivity; the most common of these is the HOMA.
It is a very simple computer model. You need only plug in is glucose and insulin. It is an on-line, Web-based computer program that anybody has access to.
Dr. Sarr: Was that done in fat people or in others?
Dr. Perugini: There were no restrictions. Indeed, the patient population that I showed you from Europe, where Stern tried to correlate the results of an insulin and glucose clamp with the HOMA, was done on a broad spectrum of the population in Europe. Does it apply to this severely obese patient population? It is not very clear. It probably does.
The final question had to do with whether these patients had central obesity versus peripheral obesity. We don’t measure that, as it is quite difficult to do reproducibly in this patient population. So, no, I have no data to correlate this with waist-to-hip circumference or central versus peripheral obesity.
Rights and permissions
About this article
Cite this article
Perugini, R.A., Quarfordt, S.H., Baker, S. et al. Metabolic Characterization of Nondiabetic Severely Obese Patients Undergoing Roux-en-Y Gastric Bypass: Preoperative Classification Predicts the Effects of Gastric Bypass on Insulin–Glucose Homeostasis. J Gastrointest Surg 11, 1083–1090 (2007). https://doi.org/10.1007/s11605-007-0158-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0158-3