Abstract
Purpose
We evaluated the diagnostic performance of a clinically available deep learning-based computer-assisted diagnosis software for detecting unruptured aneurysms (UANs) using magnetic resonance angiography and assessed the functionality of the convolutional neural network (CNN) final layer score for distinguishing between UAN and infundibular dilatation (ID).
Materials and methods
EIRL brain aneurysm (EIRL_BA) was used in this study. The subjects were 117 UAN and/or ID cases including 100 UAN lesions (average sizes of 2.56 ± 1.45 mm) and 40 ID lesions (average sizes of 1.75 ± 0.41 mm) in any of internal carotid artery, middle cerebral artery, and anterior communicating artery, and 123 normal controls. The sensitivity, specificity, and accuracy of EIRL_BA were determined for UAN and ID or UAN only. Furthermore, the relationship between the lesion category and score was examined using a linear regression analysis model, and the receiver operating characteristic (ROC) analysis was used to assess whether the scores represent UAN-like characteristics.
Results
EIRL_BA showed a total of 203 candidates (an average of 1.73/case) in UAN and/or ID cases and 98 candidates (an average of 0.80/case) in normal controls. For diagnosing either UAN/ID, EIRL_BA showed an overall sensitivity of 80%, specificity of 84.2%, and accuracy of 83.7%, resulting in the positive likelihood ratio of 5.0. For diagnosing UAN only, the overall sensitivity of 89.0, specificity of 82.6%, and accuracy of 83.2% resulting in the positive likelihood ratio of 5.1. In a linear regression analysis, the scores significantly increased in the candidates’ first and second ranks in UAN (p < 0.05) but not in ID. An ROC analysis using the score for diagnosing UAN showed an area under the curve of 0.836.
Conclusion
EIRL_BA is applicable for detecting small UAN, and the CNN’s final layer scores may be an effective index for discriminating UAN and ID and representing the likelihood of UAN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Japan, “Brain dock” started in 1988 and has been practiced in many medical institutions since. It is a voluntary examination using magnetic resonance imaging and magnetic resonance angiography (MRA) to inspect the brain exhaustively, including the investigation whether unruptured aneurysms (UAN) exist or not [1]. One of the reasons for the popularity of “brain dock” is because the incidence of a rupture of UAN that causes subarachnoid hemorrhage and frequently leads to fatal outcomes [2] is 2.8 times higher in Japanese than that in Westerners [3]. Therefore, accurate diagnosis of UAN by MRA is a crucial issue for diagnostic physicians.
However, the sensitivity of 1.5 T MRA to detect UAN is as low as 60–79% of digital subtraction angiography, which is the gold standard, with particularly low sensitivity in the regions of the internal carotid artery with less than 3 mm and the anterior cerebral artery [4]. Moreover, differentiation of infundibular dilatations (ID), initially reported as funnel-shaped dilatations at the origin of the posterior communicating artery [5, 6] is sometimes difficult because of their morphological resemblance, despite the importance of accurate diagnosis due to the distinctive follow-up strategies [7, 8].
In this situation, the development of computer-assisted diagnosis (CAD) for detecting intracranial UAN on MRA has been attempted [9,10,11,12,13], and a deep learning-based CAD system has been recently developed [14,15,16,17,18,19]. EIRL brain aneurysm® (EIRL_BA), approved for pharmaceutical use in 2019, is one of the deep learning-based CAD systems using a convolutional neural network (CNN). It is based on the algorithm where the shape and curvature of the vascular signal on the original MRA images are quantified for determining aneurysmal candidates [10]. Therefore, the scores of the CNN’s final layer, that is, the final results of the algorithm, are derived from the index of the morphological characteristics of the disease and are supposed to be able to use as the confidence level of CAD [20]. However, the validity of these scores has not been fully examined.
This study aims to evaluate the diagnostic performance of a clinically available CAD software for detecting UAN with a special interest in distinguishing UAN and ID.
Materials and methods
Ethics statement
This retrospective study was approved by the ethics committee of our hospital (No.1825). Written informed consent was waived due to the retrospective nature of the study.
Subjects
The subjects included men/women who underwent “brain dock” in our hospital from October 2017 to March 2019. In this retrospective study, subjects were picked up from the “brain dock” examinees’ database which linked to a simple diagnostic name. First, 120 cases with a diagnostic name “aneurysm” or “infundibulum” and 120 cases with age-matched “normal” cases were extracted. However, there was no record of the location or size of the lesion, and a sole reader diagnosed some cases. Therefore, two board-certified general radiologists (M.I., with 32 years of experience, M.S., with 20 years of experience, respectively) reviewed the images again. When the opinions of the two radiologists differed, the final diagnosis was determined by a consensus. As a result of a review by two radiologists, among the 120 cases that had been labeled with the diagnostic name “normal,” UAN or ID was found in 29 cases, and among the 120 cases with a diagnosis of “UAN/ID,” 32 cases did not have lesions in the target vascular regions. Therefore, the subject sample was ultimately divided into two groups: the group with cerebral aneurysm or ID in the left and/or right internal carotid arteries, the middle cerebral artery (M1–M2), or the anterior communicating artery, consisting of 117 cases with 140 lesions; and the normal group, consisting of 123 cases.
Finally, 240 cases were divided into 117 cases with 140 lesions having UAN and/or ID in either the internal carotid artery (ICA), middle cerebral artery (MCA; M1–M2), or anterior communicating artery (A-com) (= UAN/ID group), and 123 normal cases having no lesions in the three vessels (= normal control group).
The diagnostic criterion for UAN was a dome-shaped ridge protruding from the vessel in the artery wall or vascular branch which was observable by maximum intensity projection (MIP) images of at least two of three directions. The diagnostic criterion for ID was a funnel-shaped structure of < 3 mm at the bifurcation, without an aneurysmal neck, with peripheral blood vessels observed at its tip according to the definition [8]. However, we diagnosed ID not limited to the ICA, because it was often observed in other vascular areas. The source images of MRA were evaluated if necessary.
MRA image acquisition in the “Brain Dock”
All the MRA examinations were performed on 1.5 T equipment (Philips Ingenia, Philips Medical Systems, Netherlands) with a dStream Headspine coil. 3D Time-of-flight MRA data were obtained from the cranial base to the main trunk of MCA under the following parameters: repetition time/echo time 24/6.91 ms, flip angle 18°, a field of view 200 × 200 mm, acquisition matrix 352 × 223, and slice thickness 0.5 mm. MIP images were reconstructed from the original MRA images with every 15° of transverse, left and right, and coronal direction.
Analysis of MRA images by EIRL_BA
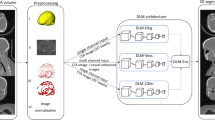
A clinically available deep learning-based CAD software (EIRL brain aneurysm (EIRL_BA), version 1.7, LPIXEL Inc., Tokyo, Japan) was used to evaluate UAN/ID. In the current version, the algorithm consists of a two-stage CNN architecture.
Analysis of MRA images by EIRL_BA was as follows (Fig. 1). First, the signal of the original MRA images is normalized and vessel extraction is performed. Next, for each pixel, the principal curvatures and the shape index are computed based on the curvature-based enhanced display system for detecting lesions in MRA [10]. The pixels for which any of these values above a certain threshold are selected as “key-points.” A 3D patch surrounding each key-point is then extracted and input into the first CNN (ResNet18). The output of the CNN is a continuous number between 0.0 and 1.0. In the first stage of CNN, key-points with an output below 0.55 are excluded, and the remaining key-points are clustered. The threshold of the first level CNN was kept to the original value validated by Ueda et al. [17]. A 3D patch around each key-point of the remaining clusters is extracted and input into the second stage of CNN (an ensemble of ten CNNs with a sigmoid activation function). The threshold of the second-stage CNN was tuned to 0.27 to reduce the number of false positives, while keeping the sensitivity the same as in the previous software version. Key-points below 0.27 were excluded. Many key-points still remained, and the remaining key-points were again clustered to confirm the scores of CNN’s final layer of the candidate and their locations were the centroid of key-points in the cluster. The clusters with the highest score are considered most similar to UAN. Up to four lesion candidates are determined based on the scores of CNN’s final layer in descending order. No candidate is displayed if no cluster has a score above the exclusion threshold.
Overview of the algorithm and two-stage CNN architecture in EIRL brain aneurysm® to detect unruptured aneurysm. 1 Each bright blue dot represents a single “key-point” (that is, a point for which the principal curvature and shape index of the MRA data are above a specific threshold). A 3D patch represented by a red wireframe box is extracted around each key-point. 2 Extracted 3D patches are fed into the first stage CNN (ResNet18). 3 The output of the first stage CNN yields a value between 0 and 1 corresponding to each key-point, and 3D patches around key-points with the first CNN’s output value above 0.55 are fed into the second-stage CNN (an ensemble of 10 CNNs). 4 Key-points (for which the average of the output of the 10 CNNs is above 0.27) are again clustered to compute the CNN’s final layer score of the candidate. CNN convolution neural network, MRA magnetic resonance angiography
EIRL-BA was connected to the PACS (picture archiving and communication system) in our hospital. The original MRA images were analyzed using the above procedure to determine the candidates’ presence and rank, and the CNN’s final layer score in the following vascular areas, including left and right ICA, left and right MCA, and A-com.
Statistical analysis
Candidates detected by EIRL_BA were assigned to one of the two lesion types (UAN and ID), which derived from the diagnosis of two board-certified radiologists (M.I., with 32 years of experience, M.S., with 20 years of experience, respectively) as the gold standard, or normal (false positive). Then, the positive diagnostic rate of EIRL_BA was calculated using a quadrant table for each vascular region. Also, since a candidate is depicted as a circle of 10 mm in diameter, EIRL-BA detection was considered accurate if the lesion diagnosed by the radiologist was within or on the circle. However, since scores were represented only in the MRA original images, in some cases, it was hard to tell whether the candidate points matched the lesion diagnosed by the radiologist, and if they were not far apart, the lesion was diagnosed as a match. Also, the candidate points of EIRL_BA that matched the radiologists’ diagnosis, the false positive candidate points in the regional vessels, and the candidate points in the regional vessels in the normal group (all false positives) were then tabulated for each candidate point rank, and the median score was determined. Distribution of the numbers and median scores of the candidates in UAN, ID lesions, and normal were aggregated for each candidate level.
The diagnostic ability of EIRL_BA was shown in a quadrant table for each vascular area. When multiple candidates were shown in one vascular area, it was considered accurate if the true candidate was included. Then, a linear regression analysis model was used to predict scores based on the lesion types in each candidate’s rank and see if UAN or ID significantly increased scores when normal scores were used as a reference. The scores were not compared between the groups, because they did not show Gaussian distribution. Candidates 3 and 4 were calculated together because candidate 4, the lowest score among the candidates, had only one UAN, no ID, and eight false positive lesions.
Finally, a receiver operating characteristic (ROC) analysis was performed with the scores of the candidates diagnosed as UAN to assess whether the score of our data represents the likelihood of UAN accurately. Statistical analyses were performed using Excel (Microsoft Corporation, Redmond, WA, USA) and Stata 17 (StataCorp LLC, College Station, TX, USA). A p value of < 0.05 was considered statistically significant.
Results
The subject characteristics of the UAN/ID and normal control groups are summarized in Table 1. In the UAN/ID group of 117 cases, a total of 140 lesions, including 96 cases of single lesions, 19 cases of double lesions, and 2 cases of triple lesions, were observed. In our cases, multiple lesions were not observed in a single vascular area. The average UAN (n = 100) size was 2.56 ± 1.45 mm, and 20/100 lesions were 3 mm or more. ID (n = 40) average size was 1.75 ± 0.41 mm, and all lesions were less than 3 mm by definition. The lesions were mostly located in ICA (n = 104), followed by A-com (n = 23) and MCA (n = 13).
Table 2 shows the distribution of the candidates detected by EIRL_BA. EIRL_BA detected 301 candidates in 240 cases, including 123 cases of normal control. In the UAN/ID group, there were 203 candidates in 185/585 vascular areas, resulting in an average of 1.73 candidates per case. In the normal group, there were 98 candidates in 95/615 vascular areas, resulting in an average of 0.80 candidates per case. The median score was highest in UAN candidate 1, and the median scores of UAN were higher than those of ID or false positive lesions.
The diagnostic accuracy of EIRL_BA in each vascular area is shown in Table 3. The sensitivity and specificity of EIRL_BA for detecting either UAN or ID lesions were 81.7 and 72.9% in ICA, 76.9 and 95.1% in MCA, and 73.9 and 80.2% in A-com, respectively. In total, sensitivity, specificity, and the accuracy were 80.0, 84.2, and 83.7%, resulting in the positive likelihood ratio of 5.0. Then, we evaluated the performance of EIRL_BA for diagnosing UAN only by excluding ID lesions from the true positive lesions. The sensitivity and the specificity for detecting UAN alone were 89.6 and 70.7% in ICA, 100 and 94.7% in MCA, and 80.0 and 78.7% in A-com, respectively. In total, the sensitivity and the specificity, and the accuracy for detecting UAN alone were 89.0 and 82.6%, and 83.2%, resulting in a positive likelihood ratio of 5.1.
Figure 2 shows the relationship between the scores and the lesion size. In either lesion category of the three vascular areas, no significant correlation was noted between the score and the lesion size.
Relationship between the sizes and the scores of the lesions in unruptured aneurysm and infundibular dilatation. Red circle, unruptured aneurysm (UAN) in internal carotid artery (ICA); yellow circle, UAN in middle cerebral artery (MCA); pink circle, UAN in anterior communicating artery (A-com); green square, infundibular dilation (ID) in ICA; dark blue square, ID in MCA; light blue square, ID in A-com. Scores of false-negative lesions are represented as 0.000
Table 4 shows the summary of the scores of UAN, ID, and normal (false positive) scores in a linear regression model. The score significantly increased to 0.233 (p < 0.0001, confidence interval 0.168–0.298) in the rank of candidate 1 and to 0.163 (p < 0.05 confidence interval 0.039–0.287) in the rank of candidate 2 in UAN, whereas not in ID when using the normal scores as a baseline. ROC analysis using the score for diagnosing UAN showed a favorable area under the curve (AUC) of 0.836 (Fig. 3). Examples of representative UANs and IDs analyzed by EIRL_BA are shown in Figs. 4, 5, 6, 7, 8.
60 s female with single unruptured aneurysm. EIRL brain aneurysm® detected a 4.5 mm (7 mm in angiography) unruptured aneurysm (UAN) in the right internal carotid artery (ICA) and a false positive candidate in the proximal portion of left ICA. Red circles indicate the locations of the candidates, and numbers beside red circles indicate the order and the CNN’s final layer score of the candidate. A Frontal MIP image, B Lateral MIP image, C MRA original image indicating right ICA UAN (1st candidate, score 1.000), D MRA original image with a false positive candidate (2nd candidate, score 0.401), E, F Oblique volume rendering images of 3D-CT angiography (yellow arrow; right ICA UAN). Note that “blebs” are obviously seen in volume rendering images. CNN convolution neural network, MIP maximal intensity projection, MRA magnetic resonance angiography, CT computed tomography
70 s male with double unruptured aneurysms. EIRL brain aneurysm® detected a 2.8 mm unruptured aneurysm (UAN) in anterior communicating artery (A-com) and 3.1 mm UAN in left middle cerebral artery (MCA). Red circles indicate the locations of the candidates, and numbers beside red circles indicate the order and the CNN’s final layer score of the candidate. A Front MIP image, B Lateral MIP image, C MRA original image indicating A-com UAN (1st candidate, score 1.000), D MRA original image indicating left MCA UAN (2nd candidate, score 0.999), E Frontal MIP image demonstrating double UAN lesions (white and yellow arrows), F Oblique volume rendering image of 3D-CT angiography demonstrating left MCA UAN (yellow arrow). CNN convolution neural network, MIP maximal intensity projection, MRA magnetic resonance angiography, CT computed tomography
50 s male with single unruptured aneurysm. EIRL brain aneurysm® detected a 4.2 mm unruptured aneurysm (UAN) in anterior communicating artery (A-com) and a false positive candidate in the proximal portion of left internal carotid artery (ICA). Red circles indicate the locations of the candidates, and numbers beside red circles indicate the order and the CNN’s final layer score of the candidate. A Front MIP image, B Lateral MIP image, C MRA original image with a false positive candidate (1st candidate, score 0.968), D MRA original image indicating A-com aneurysm (2nd candidate, score 0.644). Note that the signals of A-com UAN are lower than those of the surrounding vessel, E, F Frontal and inverted frontal volume rendering images of 3D-CT angiography (yellow arrow; A-com UAN). CNN convolution neural network, MIP maximal intensity projection, MRA magnetic resonance angiography, CT computed tomography
60 s female with single unruptured aneurysm and infundibular dilatation. EIRL brain aneurysm® detected a 2.9 mm unruptured aneurysm (UAN) in right middle cerebral artery (MCA) and a 1.9 mm infundibular dilatation (ID) in right internal carotid artery (ICA). Red circles indicate the locations of the candidates, and numbers beside red circles indicate the order and the CNN’s final layer score of the candidate. A Frontal MIP image, B Lateral MIP image, C MRA original image indicating right MCA UAN (1st candidate, score 0.995), D MRA original image indicating right ICA ID (2nd candidate, score 0.589), E, F Oblique and inverted oblique MIP images of MRA (yellow arrow; rt MCA UAN, white arrow; rt ICA ID). CNN convolution neural network, MIP maximal intensity projection, MRA magnetic resonance angiography
50 s female with single infundibular dilatation. EIRL brain aneurysm® detected a 2.3 mm infundibular dilatation (ID) in left internal carotid artery (ICA). Red circles indicate the locations of the candidates, and numbers beside red circles indicate the order and the CNN’s final layer score of the candidate. A Frontal MIP image, B Lateral MIP image, C Reversed frontal MIP image, D MRA original image indicating left ICA ID (1st candidate, score 0.762), E, F MRA original image demonstrating the tip of ID toward the left posterior communicating artery (yellow arrow). CNN convolution neural network, MIP maximal intensity projection, MRA magnetic resonance angiography
Discussion
In this study, we evaluated the diagnostic performance of CAD considering EIRL_BA as a single reader by comparing it with the diagnosis by the two expert radiologists, which was considered to be the gold standard. A common method of evaluating the diagnostic performance of CAD is to compare the reading accuracy made by radiologists when CAD is used and when it is not used. However, the calculation process of deep learning-based CAD is in a black box, so it is impossible to accurately know the “rationale for how CAD presented the candidates.” Therefore, even if CAD use increases the rate of correct diagnosis by physicians, it will not lead to optimal use if physicians do not know the rationale on which the candidate points were presented. Because optimal use of CAD requires the rationale of CAD detecting the candidates, we independently evaluated the diagnostic performance of the algorithm and examined whether the calculated scores of the CNN’s final layer could be used as the indication for the candidates.
First, the sensitivity for detecting UAN of EIRL_BA was as good as that in the previous reports of deep learning-based CAD systems. Our study also found EIRL-BA useful in detecting relatively small lesions without strictly distinguishing UAN and ID. In fact, the sensitivity of EIRL_BA detecting either UAN or ID was superior to that of single MRA reading for small aneurysms, especially in ICA, by a general radiologist [4]. This is important because both types of lesions should be pointed out in the brain dock, considering that ID could grow rounded, bloated, and UAN in the future [7, 8, 21].
Second, EIRL_BA achieved high specificity in every vascular region, resulting in high values of the positive likelihood ratio. Specificity has not been focused on previous algorithms for cerebral aneurysms to avoid missing abnormality; however, low specificity leads to over-diagnosis and unnecessary follow-up studies with expensive costs and subjects’ anxieties. Therefore, we believe EIRL_BA could be a reliable co-reader to give us valuable information about the disease without such inconvenience.
Finally, we investigated the CNN’s final layer scores based on the shape and curvature of the vascular signal in the original MRA images. If the clue is only the ranking of candidates, inexperienced readers may tend to have a bias: a high ranking of candidates suggests a high possibility of UAN, and a high number of candidates suggests the presence of at least one UAN lesion. However, the results of the linear regression analysis using the scores as an outcome showed that the scores significantly increased in UAN but not in ID, suggesting that the score could be useful for distinguishing between UAN and ID or false positive lesions. CNN is a type of artificial intelligence mimicking the human visual cortex and used for character recognition [22, 23]. EIRL_BA, based on CNN, was trained on the morphological characteristics of UAN but was not trained on those of ID. Therefore, it is natural that the ID scores are equal to those of false positive candidates. In routine readings using MRA, prompt decision whether the current vascular enlargement is UAN or ID is sometimes difficult. In this situation, looking at CNN’s final layer score may be useful to interpret, and referencing the score may be a way to make the right decision without being misled by the rank or number of candidates.
Furthermore, the results of the ROC analysis show the usability of the CNN’s final layer scores as a confidence factor of CAD, which suggests that the score can be used as a reasonable parameter for deep learning-based CAD. EIRL_BA may be helpful for a speedy, concurrent physicians’ reading if images can be read while looking at the score [24]. It is also important that 11% (11/100) of UANs did not enter the candidate. Further study is needed to evaluate whether or not the score contributes to increasing diagnostic accuracy when a physician reads brain MRA alone.
One of the limitations in this study is that the gold standard diagnosis is based on MRA alone. There may be a true UAN or ID lesion among the candidates considered false positive based on the diagnosis by the two radiologists despite correct detection by EIRL-BA. That needs to be verified in a future study. Also, as shown in case 6, it is possible that a low vascular signal in the original MIP image would result in a low score as well. Hence, further validation of false-negative lesions not detected by EIRL_BA is warranted. Additionally, subjects were small sample size from a single institution, especially cases in MCA or A-com were relatively few. Also, this study did not examine the posterior circulation aneurysms, despite it is thought to be one of the important risk factors of rupture [2, 3, 25, 26], which is a subject for further study.
In conclusion, EIRL_BA could be a reliable reader to give physicians valuable information about intracranial aneurysms under a situation where a speedy but accurate reading is required, like “brain dock,” and may be helpful for concurrent reading if the physician can read images while looking at the score; however, further study is needed.
References
Morita A. Value of brain dock (brain screening) system in Japan. World Neurosurg. 2019;127:502.
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10.
Greving JP, Wermer MJH, Brown Jr RD, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66.
Okahara M, Kiyosue H, Yamashita M, Nagatomi H, Hata H, Saginoya T, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images. Stroke. 2002;33:1803–8.
Saltzman GF. Infundibular widening of the posterior communicating artery studied by carotid angiography. Acta radiol. 1959;51(6):415–21.
Fox JL, Baiz TC, Jakoby RK. Differentiation of aneurysm from infundibulum of the posterior communicating artery. J Neurosurg. 1964;21:135–8.
Ebina K, Ohkuma H, Iwabuchi T. An angiographic study of incidence and morphology of infundibular dilatation of the posterior communicating artery. Neuroradiology. 1986;28:23–9.
Fischer S, Hopf N, Henkes H. Evolution from an infundibulum of the posterior communicating artery to a saccular aneurysm. Clin Neuroradiol. 2011;21(2):87–90.
Arimura H, Li Q, Korogi Y, Hirai T, Abe H, Yamashita Y, et al. Automated computerized scheme for detection of unruptured intracranial aneurysms in three-dimensional magnetic resonance angiography. Acad Radiol. 2004;11:1093–104.
Hayashi N, Masutani Y, Masumoto T, Mori H, Kunimatsu A, Abe O, et al. Feasibility of a curvature-based enhanced display system for detecting cerebral aneurysms in MR angiography. Magn Reson Med Sci. 2003;2:29–36.
Tada H, Ozawa Y, Hara T, Fujita H. Computerized detection of saccular aneurysms in intracranial three-dimensional MRA. IEICE Tech Rep. 2011;110:41–6.
Yang X, Blezek D, Cheng LTE, Ryan WJ, Kallmes DF. Bradley J Erickson computer-aided detection of intracranial aneurysms in MR angiography. J Digit Imaging. 2011;24:86–95.
Miki S, Hayashi N, Masutani Y, Nomura Y, Yoshikawa T, Hanaoka S, et al. Computer-assisted detection of cerebral aneurysms in MR angiography in a routine imager-reading environment: effects on diagnosis by radiologist. AJNR. 2016;37:1038–43.
Nakao T, Hanaoka S, Nomura Y, Sato I, Nemoto M, Miki S, et al. Deep neural network-based computer-assisted detection of cerebral aneurysms in MR angiography. J Magn Reson Imaging. 2018;47:948–53.
Sichtermann T, Faron A, Sijben R, Teichert N, Freiherr J, Wiesmann M. Deep learning-based detection of intracranial aneurysms in 3D TOF-MRA. AJNR. 2019;40:25–32.
Stember JN, Chang P, Stember DM, Liu M, Grinband J, Filippi CG, et al. Convolutional neural networks for the detection and measurement of cerebral aneurysms on magnetic resonance angiography. J Digit Imaging. 2019;32:808–15.
Ueda D, Yamamoto A, Nishimori M, Shimono T, Doishita S, Shimazaki A, et al. Deep learning for MR angiography: automated detection of cerebral aneurysms. Radiology. 2019;290:187–94.
Miki S, Nakao T, Nomura Y, Okimoto N, Nyunoya K, Nakamura Y, et al. Computer-aided detection of cerebral aneurysms with magnetic resonance angiography: usefulness of volume rendering to display lesion candidates. Jpn J Radiol. 2021;39(7):652–8.
Yasaka K, Akai H, Sugawara H, Tajima T, Akahane M, Yoshioka N, et al. Impact of deep learning reconstruction on intracranial 1.5 T magnetic resonance angiography. Jpn J Radiol. 2022;40(5):476–83.
Armato SG 3rd, Altman MB, Wilkie J, Sone S, Li F, Doi K, et al. Automated lung nodule classification following automated nodule detection on CT: a serial approach. Med Phys. 2003;30(6):1188–97.
Pereira BJ, Holanda VM, de Holanda CV, de Oliveira JG. Intracranial aneurysm arising from infundibular dilation. BMJ Case Rep. 2013;31:1–4.
Le Cun Y, Boser B, Denker JS, Henderson D, Howard RE, Hubbard W, et al. Handwritten digit recognition with a back-propagation network. Adv Neural Inf Process Syst. 1989;2:396–404.
Wataya T, Nakanishi K, Suzuki Y, Kido S, Tomiyama N. Introduction to deep learning: minimum essence required to launch a research. Jpn J Radiol. 2020;38(10):907–21.
Beyer F, Zierott L, Fallenberg EM, Juergens KU, Stoeckel J, Heindel W, et al. Comparison of sensitivity and reading time for the use of computer-aided detection of pulmonary nodules at MRCT as concurrent or second reader. Eur Radiol. 2007;17:2941–7.
Ishibashi T, Murayama Y, Urashima M, Saguchi T, Ebara M, Arakawa H, et al. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke. 2009;40(1):313–6.
Japan Investigators UCAS, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474–82.
Acknowledgements
We thank Keizaburo Moriuchi, a radiology technologist, for preparing and managing the image data for the study.
Funding
No funding was provided for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Makiko Ishihara, Masato Shiiba, Hirotaka Maruno, Masayuki Kato, Yuki Ohmoto-Sekine and Yasuyoshi Ouchi declare that they have no conflicts of interest; Choppin Antoine is an employee as a computer technologist of LPIXEL Inc.
Ethical statement
This study was approved by the local ethics review board and informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ishihara, M., Shiiba, M., Maruno, H. et al. Detection of intracranial aneurysms using deep learning-based CAD system: usefulness of the scores of CNN’s final layer for distinguishing between aneurysm and infundibular dilatation. Jpn J Radiol 41, 131–141 (2023). https://doi.org/10.1007/s11604-022-01341-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01341-7