Abstract
Purpose
Variable response to neoadjuvant chemoradiotherapy (nCRT) is observed among individuals with locally advanced rectal cancer (LARC), having a significant impact on patient management. In this work, we aimed to investigate the potential value of machine learning (ML)-based magnetic resonance imaging (MRI) radiomics in predicting therapeutic response to nCRT in patients with LARC.
Materials and methods
Seventy-six patients with LARC were included in this retrospective study. Radiomic features were extracted from pre-treatment sagittal T2-weighted MRI images, with 3D segmentation. Dimension reduction was performed with a reliability analysis, pair-wise correlation analysis, analysis of variance, recursive feature elimination, Kruskal–Wallis, and Relief methods. Models were created using four different algorithms. In addition to radiomic models, clinical only and different combined models were developed and compared. The reference standard was tumor regression grade (TRG) based on the Modified Ryan Scheme (TRG 0 vs TRG 1–3). Models were compared based on net reclassification index (NRI). Clinical utility was assessed with decision curve analysis (DCA).
Results
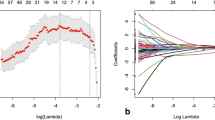
Number of features with excellent reliability is 106. The best result was achieved with radiomic only model using eight features. The area under the curve (AUC), accuracy, sensitivity, and specificity for validation were 0.753 (standard deviation [SD], 0.082), 81.1%, 83.8%, and 75.0%; for testing, 0.705 (SD, 0.145), 73.9%, 81.2%, and 57.1%, respectively. Based on the clinical only model as reference, NRI for radiomic only model was the best. DCA also showed better clinical utility for radiomic only model.
Conclusions
ML-based T2-weighted MRI radiomics might have a potential in predicting response to nCRT in patients with LARC.






Similar content being viewed by others
Abbreviations
- AJCC:
-
American joint committee on cancer
- ANN:
-
Artificial neural network
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- ICC:
-
Intra-class correlation coefficient
- LARC:
-
Locally advanced rectal cancers
- ML:
-
Machine learning
- MRI:
-
Magnetic resonance imaging
- nCRT:
-
Neoadjuvant chemoradiotherapy
- pCR:
-
Pathological complete response
- RF:
-
Random forest
- SD:
-
Standard deviation
- T2W-MRI:
-
T2-weighted MRI
- TME:
-
Total mesorectal excision
- TNM:
-
Tumor, node, metastasis
- TRG:
-
Tumor regression grade
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93.
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–83.
Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, et al. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard resection—a matched-controlled study. Dis Colon Rectum. 2017;60:1032–40.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva Sousa AH, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7.
van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet Lond Engl. 2018;391:2537–45.
Sclafani F, Brown G, Cunningham D, Wotherspoon A, Mendes LST, Balyasnikova S, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017;117:1478–85.
Curvo-Semedo L, Lambregts DMJ, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy–conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260:734–43.
Ha HI, Kim AY, Yu CS, Park SH, Ha HK. Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol. 2013;23:3345–53.
Intven M, Reerink O, Philippens MEP. Dynamic contrast enhanced MR imaging for rectal cancer response assessment after neo-adjuvant chemoradiation. J Magn Reson Imaging JMRI. 2015;41:1646–53.
Nougaret S, Vargas HA, Lakhman Y, Sudre R, Do RKG, Bibeau F, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology. 2016;280:446–54.
Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116–25.
Cai P-Q, Wu Y-P, An X, Qiu X, Kong L-H, Liu G-C, et al. Simple measurements on diffusion-weighted MR imaging for assessment of complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2014;24:2962–70.
Hötker AM, Tarlinton L, Mazaheri Y, Woo KM, Gönen M, Saltz LB, et al. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: a comparison of morphological, volumetric and functional MRI parameters. Eur Radiol. 2016;26:4303–12.
Martens MH, Subhani S, Heijnen LA, Lambregts DMJ, Buijsen J, Maas M, et al. Can perfusion MRI predict response to preoperative treatment in rectal cancer? Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;114:218–23.
Chen Y-G, Chen M-Q, Guo Y-Y, Li S-C, Wu J-X, Xu B-H. Apparent diffusion coefficient predicts pathology complete response of rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE. 2016;11: e0153944.
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:3753–60.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures. They are data. Radiology. 2016;278:563–77.
Koçak B, Durmaz EŞ, Ateş E, Kılıçkesmez Ö. Radiomics with artificial intelligence: a practical guide for beginners. Diagn Interv Radiol Ank Turk. 2019;25:485–95.
Ryan R, Gibbons D, Hyland JMP, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–6.
Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging. 2004;22:81–91.
Shafiq-Ul-Hassan M, Zhang GG, Latifi K, Ullah G, Hunt DC, Balagurunathan Y, et al. Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys. 2017;44:1050–62.
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–7.
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: synthetic minority over-sampling technique. J Artif Intell Res. 2002;16:321–57.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak Int J Soc Med Decis Mak. 2006;26:565–74.
Song Y, Zhang J, Zhang Y-D, Hou Y, Yan X, Wang Y, et al. FeAture Explorer (FAE): a tool for developing and comparing radiomics models. PLoS ONE. 2020;15: e0237587.
Van Rossum G, Drake Jr FL. Python reference manual. Centrum voor Wiskunde en Informatica Amsterdam; 1995.
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available from: https://www.R-project.org/
Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:5256–64.
Antunes JT, Ofshteyn A, Bera K, Wang EY, Brady JT, Willis JE, et al. Radiomic features of primary rectal cancers on baseline t2-weighted MRI are associated with pathologic complete response to neoadjuvant chemoradiation: a multisite study. J Magn Reson Imaging JMRI. 2020;52:1531–41.
Wang J, Chen J, Zhou R, Gao Y, Li J. Machine learning-based multiparametric MRI radiomics for predicting poor responders after neoadjuvant chemoradiotherapy in rectal Cancer patients. BMC Cancer. 2022;22:420.
Petkovska I, Tixier F, Ortiz EJ, Golia Pernicka JS, Paroder V, Bates DD, et al. Clinical utility of radiomics at baseline rectal MRI to predict complete response of rectal cancer after chemoradiation therapy. Abdom Radiol N Y. 2020;45:3608–17.
Cusumano D, Dinapoli N, Boldrini L, Chiloiro G, Gatta R, Masciocchi C, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med (Torino). 2018;123:286–95.
Meng Y, Zhang C, Zou S, Zhao X, Xu K, Zhang H, et al. MRI texture analysis in predicting treatment response to neoadjuvant chemoradiotherapy in rectal cancer. Oncotarget. 2018;9:11999–2008.
Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, et al. MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology. 2018;287:833–43.
De Cecco CN, Ganeshan B, Ciolina M, Rengo M, Meinel FG, Musio D, et al. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Invest Radiol. 2015;50:239–45.
Zhang Z, Jiang X, Zhang R, Yu T, Liu S, Luo Y. Radiomics signature as a new biomarker for preoperative prediction of neoadjuvant chemoradiotherapy response in locally advanced rectal cancer. Diagn Interv Radiol Ank Turk. 2021;27:308–14.
van Griethuysen JJM, Lambregts DMJ, Trebeschi S, Lahaye MJ, Bakers FCH, Vliegen RFA, et al. Radiomics performs comparable to morphologic assessment by expert radiologists for prediction of response to neoadjuvant chemoradiotherapy on baseline staging MRI in rectal cancer. Abdom Radiol N Y. 2020;45:632–43.
Tang X, Jiang W, Li H, Xie F, Dong A, Liu L, et al. Predicting poor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer: Model constructed using pre-treatment MRI features of structured report template. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2020;148:97–106.
Shaish H, Aukerman A, Vanguri R, Spinelli A, Armenta P, Jambawalikar S, et al. Radiomics of MRI for pretreatment prediction of pathologic complete response, tumor regression grade, and neoadjuvant rectal score in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation: an international multicenter study. Eur Radiol. 2020;30:6263–73.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yardimci, A.H., Kocak, B., Sel, I. et al. Radiomics of locally advanced rectal cancer: machine learning-based prediction of response to neoadjuvant chemoradiotherapy using pre-treatment sagittal T2-weighted MRI. Jpn J Radiol 41, 71–82 (2023). https://doi.org/10.1007/s11604-022-01325-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01325-7