Abstract
Purpose
The aim of this study was to determine the cutoff level of apparent diffusion coefficient (ADC) values for diagnosing prostate cancer.
Materials and methods
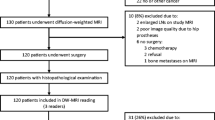
A total of 45 consecutive patients with prostate cancer who underwent diffusion-weighted magnetic resonance imaging (MRI) with ADC maps before radical prostatectomy were included in this retrospective study. MRI findings were correlated retrospectively with histopathological results of surgical specimens. Comparisons of ADC values between cancer and noncancer areas were performed with the two-tailed unequal variance t-test. The cutoff ADC level was determined in a way to achieve the best accuracy for detecting prostate cancer.
Results
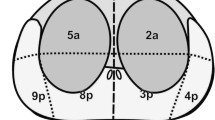
The mean ADC value of all the cancer lesions (n =60) was 1.04 ± 0.31 (×10−3 mm2/s). In the peripheral zone, the mean ADC values of cancer lesions and noncancer areas were 1.07 ± 0.35 and 1.94 ± 0.31, respectively (P < 0.001). In the transition zone, the mean ADC values of cancer lesions and noncancer areas were 1.00 ± 0.22 and 1.56 ± 0.14, respectively (P<0.001). The cutoff level for the ADC value was determined to be 1.35×10−3 mm2/s. It provided sensitivity, specificity, and accuracy of 88%, 96%, and 93%, respectively.
Conclusion
The cutoff ADC level determined on the basis of the results obtained from radical prostatectomy specimens can help differentiate malignant from nonmalignant lesions.
Similar content being viewed by others
References
Bloch BN, Furman-Haran E, Helbich TH, Lenkinski RE, Degani H, Kratzik C, et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging: initial results. Radiology 2007;245:176–185.
Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology 2007;243:28–53.
Kim JK, Hong SS, Choi YJ, Park SH, Ahn H, Kim CS, et al. Wash-in rate on the basis of dynamic contrast-enhanced MRI: usefulness for prostate cancer detection and localization. J Magn Reson Imaging 2005;22:639–646.
Tamada T, Sone T, Jo Y, Yamamoto A, Yamashita T, Egashira N, et al. Prostate cancer: relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology 2008;248:531–539.
Langer DL, van der Kwast TH, Evans AJ, Trachtenberg J, Wilson BC, Haider MA. Prostate cancer detection with multi-parametric MRI: logistic regression analysis of quantitative T2, diffusion-weighted imaging, and dynamic contrast-enhanced MRI. J Magn Reson Imaging 2009;30:327–334.
Lovett K, Rifkin MD, McCue PA, Choi H. MR imaging characteristics of noncancerous lesions of the prostate. J Magn Reson Imaging 1992;2:35–39.
Naik KS, Carey BM. The transrectal ultrasound and MRI appearances of granulomatous prostatitis and its differentiation from carcinoma. Clin Radiol 1999;54:173–175.
Oyen RH, Van de Voorde WM, Van Poppel HP, Brys PP, Ameye FE, Franssens YM, et al. Benign hyperplastic nodules that originate in the peripheral zone of the prostate gland. Radiology 1993;189:707–711.
Shukla-Dave A, Hricak H, Eberhardt SC, Olgac S, Muruganandham M, Scardino PT, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings: initial observations. Radiology 2004;231:717–724.
White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, Zaloudek CJ, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology 1995;195:385–390.
Ikonen S, Kivisaari L, Tervahartiala P, Vehmas T, Taari K, Rannikko S. Prostatic MR imaging: accuracy in differentiating cancer from other prostatic disorders. Acta Radiol 2001;42:348–354.
Noguchi M, Stamey TA, Neal JE, Yemoto CE. An analysis of 148 consecutive transition zone cancers: clinical and histological characteristics. J Urol 2000;163:1751–1755.
Ito H, Kamoi K, Yokoyama K, Yamada K, Nishimura T. Visualization of prostate cancer using dynamic contrast-enhanced MRI: comparison with transrectal power Doppler ultrasound. Br J Radiol 2003;76:617–624.
Zakian KL, Eberhardt S, Hricak H, Shukla-Dave A, Kleinman S, Muruganandham M, et al. Transition zone prostate cancer: metabolic characteristics at 1H MR spectroscopic imaging: initial results. Radiology 2003;229:241–247.
Gibbs P, Tozer DJ, Liney GP, Turnbull LW. Comparison of quantitative T2 mapping and diffusion-weighted imaging in the normal and pathologic prostate. Magn Reson Med 2001;46:1054–1058.
Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, et al. Combined T2-weighted and diffusion- weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 2007;189:323–328.
Hosseinzadeh K, Schwarz SD. Endorectal diffusion-weighted imaging in prostate cancer to differentiate malignant and benign peripheral zone tissue. J Magn Reson Imaging 2004;20:654–661.
Issa B. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J Magn Reson Imaging 2002;16:196–200.
Kitajima K, Kaji Y, Kuroda K, Sugimura K. High b-value diffusion-weighted imaging in normal and malignant peripheral zone tissue of the prostate: effect of signal-to-noise ratio. Magn Reson Med Sci 2008;7:93–99.
Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis: correlation with biopsy and histopathology. J Magn Reson Imaging 2006;24:108–113.
Langer DL, van der Kwast TH, Evans AJ, Sun L, Yaffe MJ, Trachtenberg J, et al. Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2-sparse versus dense cancers. Radiology 2008;249:900–908.
Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection: a multireader study. Radiology 2009;250:145–151.
Sato C, Naganawa S, Nakamura T, Kumada H, Miura S, Takizawa O, et al. Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging 2005;21:258–262.
Tamada T, Sone T, Jo Y, Toshimitsu S, Yamashita T, Yamamoto A, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging 2008;28:720–726.
Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging 2007;25:146–152.
Yoshimitsu K, Kiyoshima K, Irie H, Tajima T, Asayama Y, Hirakawa M, et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging 2008;27:132–139.
Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 2006; 24:478–488.
Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217:331–345.
Murtz P, Flacke S, Traber F, van den Brink JS, Gieseke J, Schild HH. Abdomen: diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology 2002;224:258–264.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216.
Toulon P, Smirnov M, Triscott M, Safa O, Biguzzi E, Bouziane K, et al. A new chromogenic assay (HemosIL ThromboPath) is sensitive to major prothrombotic risk factors affecting the protein C pathway: results of a multicenter study. Thromb Res 2009;124:137–143.
Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 2000;43:828–836.
Mcneal JE. Anatomy and normal histology of the human prostate. In: Foster CS, Bostwick DG, editors. Pathology of the prostate. Philadelphia: Saunders; 1998. p.19–34.
Ishida J, Sugimura K, Okizuka H, Kaji Y, Moriyama M, Nagaoka S, et al. Benign prostatic hyperplasia: value of MR imaging for determining histologic type. Radiology 1994;190:329–331.
Kim CK, Park BK, Han JJ, Kang TW, Lee HM. Diffusionweighted imaging of the prostate at 3 T for differentiation of malignant and benign tissue in transition and peripheral zones: preliminary results. J Comput Assist Tomogr 2007;31:449–454.
Mazaheri Y, Hricak H, Fine SW, Akin O, Shukla-Dave A, Ishill NM, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology 2009; 252:449–457.
Outwater E, Schiebler ML, Tomaszewski JE, Schnall MD, Kressel HY. Mucinous carcinomas involving the prostate: atypical findings at MR imaging. J Magn Reson Imaging 1992;2:597–600.
Montironi R, Mazzuccheli R, Scarpelli M, Lopez-Beltran A, Fellegara G, Algaba F. Gleason grading of prostate cancer in needle biopsies or radical prostatectomy specimens: contemporary approach, current clinical significance and sources of pathology discrepancies. BJU Int 2005;95:1146–1152.
Akin O, Sala E, Moskowitz CS, Kuroiwa K, Ishill NM, Pucar D, et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology 2006;239:784–792.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nagayama, M., Watanabe, Y., Terai, A. et al. Determination of the cutoff level of apparent diffusion coefficient values for detection of prostate cancer. Jpn J Radiol 29, 488–494 (2011). https://doi.org/10.1007/s11604-011-0586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-011-0586-6