Summary
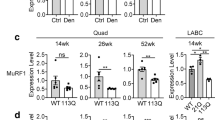
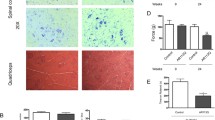
In polyglutamine (PolyQ) diseases, mutant proteins cause not only neurological problems but also peripheral tissue abnormalities. Among all systemic damages, skeletal muscle dystrophy is the severest. Previously by studying knock-in (KI) mouse models of spinal cerebellar ataxia 17 (SCA17), it was found that mutant TATA box binding protein (TBP) decreases its interaction with myogenic differentiation antigen, thus reducing the expression of skeletal muscle structural proteins and resulting in muscle degeneration. In this paper, the role of mutant TBP in myogenesis was investigated. Single myofibers were isolated from tibialis anterior muscles of wild type (WT) and SCA17KI mice. The 1TBP18 staining confirmed the expression of mutant TBP in muscle satellite cells in SCA17KI mice. In the BaCl2-induced TA muscle injury, H&E cross-section staining showed no significant change in myofibril size before and after BaCl2 treatment, and there was no significant difference in centralized nuclei between WT and SCA17KI mice, suggesting that mutant TBP had no significant effect on muscle regeneration. In the cultured primary myoblasts from WT and SCA17KI mice in vitro, representative BrdU immunostaining showed no significant difference in proliferation of muscle satellite cells. The primary myoblasts were then induced to differentiate and immunostained for eMyHC, and the staining showed there was no significant difference in differentiation of primary myoblasts between WT and SCA1KI mice. Our findings confirmed that mutant TBP had no significant effect on myogenesis.
Similar content being viewed by others
References
Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci, 2007,30:575–621
Bates GP, Dorsey R, Gusella JF, et al. Huntington disease. Nat Rev Dis Primers, 2015,1:15005
Paulson HL, Shakkottai VG, Clark HB, et al. Polyglutamine spinocerebellar ataxias— from genes to potential treatments. Nat Rev Neurosci, 2017,18(10):613–626
Martin B, Golden E, Keselman A, et al. Therapeutic perspectives for the treatment of Huntington’s disease: treating the whole body. Histol Histopathol, 2008,23(2): 237–250
Carroll JB, Bates GP, Steffan J, et al. Treating the whole body in Huntington’s disease. Lancet Neurol, 2015, 14(11):1135–1142
Cortes CJ, Ling SC, Guo LT, et al. Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron, 2014,82(2):295–307
Lim J, Crespo BJ, Jafar NP, et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature, 2008,452(7188):713–718
Aladão PA, de Aragão BC, Andrade JN, et al. Muscle atrophy is associated with cervical spinal motoneuron loss in BACHD mouse model for Huntington’s disease. Eur J Neurosci, 2017,45(6):785–796
van Roon-Mom WM, Reid SJ, Faull RL, et al. TATA binding protein in neurodegenerative disease. Neuroscience, 2005,133(4):863–872
Vargas AP, Carod-Artal FJ, Bomfim D, et al. Unusual early-onset Huntingtons disease. J Child Neurol, 2003, 18(6):429–432
Huang S, Yang S, Guo J, et al. Large Polyglutamine Repeats Cause Muscle Degeneration in SCA17 Mice. Cell Rep, 2015,13(1):196–208
Forcina L, Miano C, Pelosi L. An Overview about the Biology of Skeletal Muscle Satellite Cells. Curr Genomics, 2019,20(1):24–37
Huang S, Ling JJ, Yang S, et al. Neuronal expression of TATA box-binding protein containing expanded polyglutamine in knock-in mice reduces chaperone protein response by impairing the function of nuclear factor-Y transcription factor. Brain, 2011,134(Pt7):1943–1958
Friedman MJ, Shah AG, Fang ZH, et al. Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration. Nat Neurosci, 2007,10(12):1519–1528
Mitchell PO, Mills ST, O’Connor RS, et al. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev Biol, 2005,283(1):240–252
Horsley V, Pavlath GK. Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2 dependent pathway. J Cell Biol, 2003,161(1):111–118
Apponi LH, Leung SW, Williams KR, et al. Loss of nuclear poly(A)-binding protein 1 causes defects in myogenesis and mRNA biogenesis. Hum Mol Genet, 2010,19(6):1058–1065
Kong D, He M, Yang L, et al. MR-17 and miR-19 cooperatively promote skeletal muscle cell differentiation. Cell Mol Life Sci, 2019, Jun. 18. [Epub ahead of print] doi:https://doi.org/10.1007/s00018-019-03165-7
Huang S, Zhu S, Li XJ. The Expanding Clinical Universe of Polyglutamine Disease. Neuroscientist, 2019, Jan.7. [Epub ahead of print] doi:https://doi.org/10.1177/1073858418822993
Zielonka D, Piotrowska I, Marcinkowski JT. Skeletal muscle pathology in Huntington’s disease. Front Physiol, 2014,5:380
Giorgetti E. Polyglutamine androgen receptor-mediated neuromuscular disease. Cell Mol Life Sci, 2016,73(21): 3991–3999
Dupuis L. Skeletal muscle in motor neuron diseases: therapeutic target and delivery route for potential treatments. Curr Drug Targets, 2010,11(10):1250–1261
Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol, 1994,125(6): 1275–1287
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This project was supported by grants from the fundamental Research Funds for the Central Universities (No. 2019kfyXKJC075), National Key R&D Program of China (No. 2017YFC1310000) and National Natural Science Foundation of China (No. 81671064, and No. 81371222).
Rights and permissions
About this article
Cite this article
Zhao, Dm., Zhu, Sq., Wang, Fr. et al. Role of Mutant TBP in Regulation of Myogenesis on Muscle Satellite Cells. CURR MED SCI 39, 734–740 (2019). https://doi.org/10.1007/s11596-019-2099-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-019-2099-y