Abstract
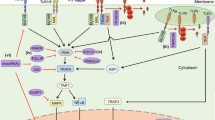
Keloid may induce severe impairment of life quality for the patients, although keloid is a cutaneous benign tumor. Collagen triple helix repeat containing protein 1 (Cthrc1) was identified as a novel gene that was originally found in adventitial fibroblasts after arterial injury. To address the role of Cthrc1 in keloid, the expression level of Cthrc1 was assessed in normal skin and keloid tissue, as well as in normal fibroblasts (NFs) and keloid fibroblasts (KFs) by using quantitative PCR, Western blotting and immunohistochemical analysis. The results showed that Cthrc1 was increased in keloid tissue and KFs as compared with normal skin and NFs. Meanwhile, CCK8 and Transwell assays found the cellular proliferation and migration of KFs were increased as compared with NFs. Further, to verify the function of Cthrc1 in NFs and KFs, we increased Cthrc1 expression by transfecting lentivirus (LV) vectors LV-Cthrc1. The cellular proliferation and migration, collagen synthesis and the influence on TGF-β and YAP signaling were tested. The cellular proliferation and migration were increased in NFs-Cthrc1 as compared with NFs-control. Meanwhile, YAP expression and nuclear-location was increased in NFs-Cthrc1. On the contrary, when Cthrc1 was overexpressed in KFs, the cellular migration was suppressed and YAP expression was reduced and transferred to cytoplasm in KFs-Cthrc1 as compared with KFs-control. But the expression level of collagen I was decreased and pSmad2/3 nucleus transfer was suppressed in both NFs-Cthrc1 and KFs-Cthrc1 by using Western blotting and immunofluorescence. Increased Cthrc1 activated NFs by promoting YAP nucleus translocation, whereas suppressed KFs by inhibiting YAP nucleus translocation. Enhanced Cthrc1 decreased collagen I in both NFs and KFs by inhibiting TGF-β/Smad pathway. In conclusion, Cthrc1 may play a role in the pathogenesis of keloid by inhibiting collagen synthesis and fibroblasts migration via suppressing TGF-β/Smad pathway and YAP nucleus translocation.
Similar content being viewed by others
References
Arno AI, Gauglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns, 2014,40(7):1255–1266
Liu F, Lagares D, Choi KM, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol, 2014,308(4):L344–L357
Pyagay P, Heroult M, Wang Q, et al. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res, 2005,96(2):261–268
Durmus T, LeClair RJ, Park KS, et al. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1). Gene Expr Patterns, 2006,6(8):935–940
LeClair R, Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc Med, 2007,17(6):202–205
Bian Z, Miao Q, Zhong W, et al. Treatment of cholestatic fibrosis by altering gene expression of Cthrc1: Implications for autoimmune and nonautoimmune liver disease. J Autoimmun, 2015,63:76–87
Unahabhokha T, Sucontphunt A, Nimmannit U, et al. Molecular signalings in keloid disease and current therapeutic approaches from natural based compounds. Pharm Biol, 2015,53(3):457–463
LeClair RJ, Durmus T, Wang Q, et al. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circ Res, 2007,100(6):826–833
Li J, Cao J, Li M, et al. Collagen triple helix repeat containing-1 inhibits transforming growth factor-β1-induced collagen type I expression in keloid. Br J Dermatol. 2011,164(5):1030–1036
Lee MJ, Byun MR, Furutani-Seiki M, et al. YAP and TAZ Regulate Skin Wound Healing. J Invest Dermatol, 2014,134(2):518–525
Zhao B, Li L, Lu Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev, 2011,25(1):51–63
Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature, 2013,493(7430):106–110
Liu M, Zou W, Huang M, et al. Isolation and cultivation of bovine corneal stromal fibroblasts by two-step collagenase digestion method. Zhongguo Zuzhi Gongcheng Yanjiu (Chinese). 2012,16(7):1201–1205
Ke Z, He W, Lai Y, et al. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget, 2014,5(19):9410–9424
Tan F, Liu F, Liu H, et al. CTHRC1 is associated with peritoneal carcinomatosis in colorectal cancer: a new predictor for prognosis. Med Oncol, 2013,30(1):473
Hou M, Cheng Z, Shen H, et al. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget, 2015,6(34):35813–35829
Wang L, Xiang YN, Zhang YH, et al. Collagen triple helix repeat containing-1 in the differential diagnosis of dermatofibrosarcoma protuberans and dermatofibrom. Br J Dermatol, 2011,164(1):135–140
Ip W, Wellman-Labadie O, Tang L, et al. Collagen triple helix repeat containing 1 promotes melanoma cell adhesion and survival. J Cutan Med Surg, 2011,15(2):103–110
Kharaishvili G, Magdalena C, Katerina B, et al. Collagen triple helix repeat containing 1 protein, periostin and versican in primary and metastatic breast cancer: an immunohistochemical study. J Clin Pathol, 2011,64(11):977–982
Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature, 2008,453(7193):314–321
Liu X, He H, Yin JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int, 2006,26(1):8–22
Mehal WZ, Iredale J, Friedman SL. Scraping fibrosis: expressway to the core of fibrosis. Nat Med, 2011,17(5):552–553
Denton CP, Merkel PA, Furst DE, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum, 2007,56(1):323–333
Wang L, Xiang YN, Zhang YH, et al. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-beta1 and may be associated with metastasis in human gastric cancer. Cancer Sci, 2012,103(7):1327–1333
Piersma B, Bank RA, Boersema M. Signaling in fibrosis: TGF-beta, WNT, and YAP/TAZ converge. Front Med (Lausanne), 2015,2:59
Varelas X, Wrana JL. Coordinating developmental signaling: novel roles for the Hippo pathway. Trends Cell Biol, 2012,22(2):88–96
Preisser F, Giehl K, Rehm M, et al. Inhibitors of oxygen sensing prolyl hydroxylases regulate nuclear localization of the transcription factors Smad2 and YAP/TAZ involved in CTGF synthesis. Biochim Biophys Acta, 2016,1863(8):2027–2036
Grannas K, Arngarden L, Lonn P, et al. Crosstalk between Hippo and TGFβ: subcellular localization of YAP/TAZ/Smad complexes. J Mol Biol, 2015,427(21):3407–3415
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the National Natural Science Foundation of China (No. 81472886 and No. 81172588).
Rights and permissions
About this article
Cite this article
Zhao, Mj., Chen, Sy., Qu, Xy. et al. Increased Cthrc1 Activates Normal Fibroblasts and Suppresses Keloid Fibroblasts by Inhibiting TGF-β/Smad Signal Pathway and Modulating YAP Subcellular Location. CURR MED SCI 38, 894–902 (2018). https://doi.org/10.1007/s11596-018-1959-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1959-1