Summary
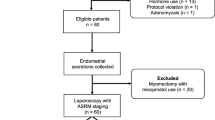
The aim of this study was to investigate the expression of macrophage migration inhibitory factor (MMIF), hypoxia-inducible factor-1 a (HIF-1α) and vascular endothelial growth factor (VEGF) in the serum and endometrial tissues of patients with endometriosis (EM) and the clinical significance. Eighty EM patients [American Reproductive Association stage I (n=20), stage II (n=22), stage III (n=21) and stage IV (n=17)] were enrolled and divided into mild (10-14 points, n=28), moderate (16-24 points, n=27) and severe (26-30 points, n=25) dysmenorrhea groups. The control group included 40 healthy women of childbearing age who underwent routine healthcare examinations in the enrolment period. The expression of MMIF, HIF-1α and VEGF in the serum and endometrial tissues was measured by enzyme-linked immunosorbent assay and Western blotting, respectively. Meanwhile, the sensitivity and specificity of serum MMIF, HIF-1α, and VEGF when separately used as single indexes or jointly used as one index were examined as well. The results showed that serum concentrations of MMIF, HIF-1α, and VEGF were significantly higher in EM patients than in controls (P<0.05). The expression of all three proteins in both serum and endometrial tissues increased significantly with the R-AFS stage (P<0.05) and with dysmenorrheal severity (P<0.05). The sensitivity and specificity of the combined detection of serum MMIF, HIF-1α, and VEGF levels were significantly higher than those of single index detection (P<0.05). In conclusion, the expression of MMIF, HIF-1α, and VEGF in the serum and endometrial tissues may be used to assess the stage of EM and the severity of dysmenorrhea. Combined evaluation of MMIF, HIF-1α, and VEGF significantly improves the diagnostic sensitivity and specificity.
Similar content being viewed by others
References
Psaroudakis D, Hirsch M, Davis C. Review of the management of ovarian endometriosis: paradigm shift towards conservative approaches. Curr Opin Obstet Gynecol, 2014,26(4):266–274
Dancet EA, Apers S, Kremer JA, et al. The patientcenteredness of endometriosis care and targets for improvement: a systematic review. Gynecol Obstet Invest, 2014,78(2): 69–80
Veillat V, Sengers V, Metz CN, et al. Macrophage migration inhibitory factor is involved in a positive feedback loop increasing aromatase expression in endometriosis. Am J Pathol, 2012,181(3):917–927
Serrano-Oviedo L, Giménez-Bachs JM, Nam-Cha SY, et al. Implication of VHL, ERK5, and HIF-1 alpha in clear cell renal cell carcinoma: Molecular basis. Urol Oncol, 2017,35(3):el5–e22
Xu H, Zhang T, Man GC, et al. Vascular endothelial growth factor C is increased in endometrium and promotes endothelial functions, vascular permeability and angiogenesis and growth of endometriosis. Angiogenesis, 2013,16(3):541–551
Stilley JA, Birt JA, Sharpe-Timms KL. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res, 2012,349(3):849–862
Gregoric P, Doklestic K, Pandurovic M, et al. Distal ideal endometriosis as a cause of ileus: a case report. Srp Arh Celok Lek, 2012,140(3-4):225–228
Mei J, Xie XX, Li MQ, et al. Indoleamine 2, 3-dioxygenase-l (IDOl) in human endometrial stromal cells induces macrophage tolerance through interleukin-33 in the progression of endometriosis. Int J Clin Exp Pathol, 2014,7(6):2743–2757
Pirdel L, Pirdel M. Role of iron overload-induced macrophage apoptosis in the pathogenesis of peritoneal endometriosis. Reproduction, 2014,147(6):R199–R207
Lin W, Chen S, Li M, et al. Expression of macrophage migration inhibitory factor in human endometriosis: relation to disease stage, menstrual cycle and infertility. J Obstet Gynaecol Res, 2010,36(2):344–351
Pan X, Mao X, Cheng T, et al. Macrophage migration inhibitiory factor: a regular of MMP13 and inflammation in titanium particles stimulated air pouch in vivo. Mol Cell Biochem, 2011,357(1-2):313–321
Baron N, Deuster O, Noelker C, et al. Role of macrophage migration inhibitory factor in primary glioblastoma multiforme cells. J Neurosci Res, 2011,89(5):711–717
Ajdukovic J. HIF-l-a big chapter in the cancer tale. Exp Oncol, 2016,38(1):9–12
Eslavath RK, Sharma D, Bin Omar NA, et al. P-N-oxalyl-L-a, P-diaminopropionic acid induces HRE expression by inhibiting HIF-prolyl hydroxylase-2 in normoxic conditions. Eur J Pharmacol, 2016,791:405–411
Li J, Mi C, Ma J, et al. Dihydrotanshinone I inhibits the translational expression of hypoxia-inducible factor-la. Chem Biol Interact, 2015,240(5):48–58
El-Naggar AM, Veinotte CJ, Cheng H, et al. Translational activation of HIF1α by YB-1 promotes sarcoma metastasis. Cancer Cell, 2015,27(5):682–697
Critchley HO, Osei J, Henderson TA, et al. Hypoxiainducible factor-1 alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2(EP2). Endocrinology, 2006,147(2):744–753
Goteri G, Lucarini G, Zizzi A, et al. Proangiogenetic molecules, hypoxia-inducible factor-1 alpha and nitric oxide synthase isoforms in ovarian endometriotic cysts. Virchows Arch, 2010,456(6):703–710
Wu MH, Lu CW, Chang FM, et al. Estrogen receptor expression affected by hypoxia inducible factor-la in stromal cells from patients with endometriosis. Taiwan J Obstet Gynecol, 2012,51(2):50–54
Kim KH, Kim HY, Kim HH, et al. Hypoxia induces expression of COX-2 through the homeodomain transcription factor CDX1 and orphan nuclear receptor SHP in human endometrial cells. Mol Hum Reprod, 2011,17(3):710–719
Hsiao KY, Chang N, Lin SC, et al. Inhibition of dual specificity phosphatase-2 by hypoxia promotes interleukin-8-mediated angiogenesis in endometriosis. Hum Reprod, 2014,29(2):2747–2755
Courtnay R, Ngo DC, Malik N, et al. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep, 2015,42(5):841–851
Denko NC. Hypoxia HIFI and glucose metabolism in the solid tumour. Nat Rev Cancer, 2008,8(2):705–713
Cho S, Choi YS, Jeon YE, et al. Expression of vascular endothelial growth factor (VEGF) and its soluble receptor-1 inendometriosis. Microvasc Res, 2012,83(2):237–242
Leone R Maggiore U, Ferrero S. Methodological concerns regarding levels of vascular endothelial growth factor (VEGF) in serum of women with endometriosis. Int J Fertil Steril, 2015,8(4):485–486
Fang F, Gong L, Wang X, et al. The association between vascular endothelial growth factor (VEGF) +405G>C genetic polymorphism and endometriosis. Exp Biol Med, 2015,240(9): 1177–1182
Gonçalves GA, Camargo-Kosugi CM, Bonetti TC, etal. p27kipl overexpression regulates VEGF expression, cell proliferation and apoptosis in cell culture from eutopic endometrium of women with endometriosis. Apoptosis, 2015,20(3):327–335
Huang F, Wang H, Zou Y, et al. Effect of GnRH-II on the ESC proliferation, apoptosis and VEGF secretion in patients withendometriosis in vitro. Int J Clin Exp Pathol, 2013,6(ll):2487–2496
Li YZ, Wang LJ, Li X, et al. Vascular endothelial growth factor gene polymorphisms contribute to the risk of endometriosis: an updated systematic review and meta-analysis of 14 case-control studies. Genet Mol Res, 2013,12(2): 1035–1044
Barcz E, Milewski L, Dziunycz P, et al. Peritoneal cytokines and adhesion formation in endometriosis: an inverse association with vascular endothelial growth factor concentration. Fertil Steril, 2012,97(6): 1380–1386
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Liu, Xl., Wang, W. et al. Expression of MMIF, HIF-1α and VEGF in Serum and Endometrial Tissues of Patients with Endometriosis. CURR MED SCI 38, 499–504 (2018). https://doi.org/10.1007/s11596-018-1906-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1906-1