Summary
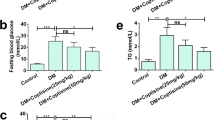
Diabetic nephropathy (DN) is a common and serious clinical complication of diabetes and presently there are no effective ways to prevent its occurrence and progression. Recent studies show that pentoxifylline (PTX) can improve renal hemodynamics, reduce urinary protein excretion, and alleviate or delay renal failure in DN patients. In this study, we focused on the anti-oxidative stress effect of PTX on alleviating renal damages of DN using rat models. DN rats were established with injection of streptozotocin. Blood glucose, urinary protein excretion, serum cystatin C, renal biopsy, superoxide dismutase (SOD) and malondialdehyde (MDA) in serum and renal homogenate and renal nitrotyrosine levels were analyzed before and 12 weeks after the treatment of PTX. Before treatment, all the DN rats had elevated blood glucose, increased urinary protein excretion and elevated serum cystatin C. Morphologically, DN rats exhibited renal tissue damages, including swelling and fusions of foot processes of podocytes under electron microscope. Masson staining revealed blue collagen deposition in glomeruli and renal interstitium. With treatment of PTX, symptoms and renal pathological changes of DN rats were alleviated. Furthermore, the MDA levels were increased and the SOD levels were decreased in the serum and kidneys of DN rats, and these changes were reversed by PTX. The expression of nitrotyrosine was up-regulated in DN rat model and down-regulated by PTX, indicating that PTX was able to inhibit oxidative reactions in DN rats. PTX could alleviate renal damage in DN, which may be attributable to its anti-oxidative stress activity.
Similar content being viewed by others
References
Fernández B, Elewa U, Sánchez-Niño MD, et al. 2012 update on diabetic kidney disease: the expanding spectrum, novel pathogenic insights and recent clinical trials. Minerva Med, 2012,103(4):219–234
Kania DS, Smith CT, Nash CL, et al. Potential new treatments for diabetic kidney disease. Med Clin North Am, 2013,97(1):115–134
Navarro-González JF, Muros M, Mora-Fernández C, et al. Pentoxifylline for renoprotection in diabetic nephropathy: the PREDIAN study. Rationale and basal results. J Diabetes Complications, 2011,25(5):314–319
Badri S, Dashti-Khavidaki S, Lessan-Pezeshki M, et al. A review of the potential benefits of pentoxifylline in diabetic and non-diabetic proteinuria. J Pharm Pharm Sci, 2011,14(1):128–137
Lin SL, Chen YM, Chien CT, et al. Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. J Am Soc Nephrol, 2002,13(12):2916–2929
Tavafi M. Diabetic nephropathy and antioxidants. J Nephropathol, 2013,2(1): 20–27
Taniguchi K, Xia L, Goldberg HJ, et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes, 2013,62(11):3874–3886
Wu J, Zhang R, Torreggiani M, et al. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol, 2010,176(5):2163–2176
Islam MS. Animal models of diabetic neuropathy: progress since 1960s. J Diabetes Res, 2013,2013:149452
Kong LL, Wu H, Cui WP, et al. Advances in murine models of diabetic nephropathy. J Diabetes Res,2013, 2013:797548.
An ZM, Dong XG. The advances in research commonly used method for on animal models of type 2 diabetic nephropathy. Med Review, 2013,29(23): 554–558
Li GS, Zeng Y, Li QY, et al. Comparison of efficacy of mycophenolate mofetil and leflumide in treatment of diabetic nephropathy in rats. J Huazhong Univ Sci Technol (Med Sci), 2013,42(6):647–650
Inker LA, Schmid CH, Tighiouart H,et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med, 2012,367(1):20–29
McCormick BB, Sydor A, Akbari A, et al. The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am J Kidney Dis, 2008,52(3):454–463
Laczy B, Cseh J, Mohás M, et al. Effects of pentoxifylline and pentosan polysulphate combination therapy on diabetic neuropathy in type 2 diabetes mellitus. Acta Diabetol, 2009,46(2):105–111
Sun HK, Lee YM, Han KH, et al. Phosphodiesterase inhibitor improves renal tubulointerstitial hypoxia of the diabetic rat kidney. Korean J Intern Med, 2012,27(2):163–170
Han KH, Han SY, Kim HS, et al. Prolonged administration enhances the renoprotective effect of pentoxifylline via anti-inflammatory activity in streptozotocin-induced diabetic nephropathy. Inflammation, 2010,33(3):137–143
Mima A. Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J Diabetes Res, 2013,2013:248563
Stanton RC. Oxidative stress and diabetic kidney disease. Curr Diab Rep, 2011,11(4):330–336
Gupta A, Gupta P, Biyani M. Targeted therapies in diabetic nephropathy: an update. J Nephrol, 2011,24(6):686–695
Zein CO, Lopez R, Fu X, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology, 2012,56(4):1291–1299
Escobar J, Pereda J, Arduini A, et al. Oxidative and nitrosative stress in acute pancreatitis. Modulation by pentoxifylline and oxypurinol. Biochem Pharmacol, 2012, 83(1):122–130
Wang GG, Lu XH, Li W, et al. Protective Effects of Luteolin on diabetic nephropathy in STZ-induced diabetic Rats. evid based Complement Alternat Med, 2011,2011:323171
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from the Research Program of Huangpu District Science and Technology Committee of Shanghai, China (No. 2012-HGG-5), and the Program for Outstanding Academic Leaders of Health System of Huangpu District of Shanghai, China (No. 2013-18).
Rights and permissions
About this article
Cite this article
An, Zm., Dong, Xg., Guo, Y. et al. Effects and clinical significance of pentoxifylline on the oxidative stress of rats with diabetic nephropathy. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 35, 356–361 (2015). https://doi.org/10.1007/s11596-015-1437-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-015-1437-y