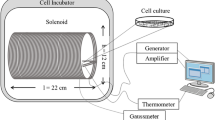
Summary
This study examined the osteogenic effect of electromagnetic fields (EMF) under the simulated in vivo conditions. Rat bone marrow mesenchymal stem cells (BMSCs) and rat osteoblasts were co-cultured and exposed to 50 Hz, 1.0 mT EMF for different terms. Unexposed single-cultured BMSCs and osteoblasts were set as controls. Cell proliferation features of single-cultured BMSCs and osteoblasts were studied by using a cell counting kit (CCK-8). For the co-culture system, cells in each group were randomly chosen for alkaline phosphatase (ALP) staining on the day 7. When EMF exposure lasted for 14 days, dishes in each group were randomly chosen for total RNA extraction and von Kossa staining. The mRNA expression of osteogenic markers was detected by using real-time PCR. Our study showed that short-term EMF exposure (2 h/day) could obviously promote proliferation of BMSCs and osteoblasts, while long-term EMF (8 h/day) could promote osteogenic differentiation significantly under co-cultured conditions. Under EMF exposure, osteogenesis-related mRNA expression changed obviously in co-cultured and single-cultured cells. It was noteworthy that most osteogenic indices in osteoblasts were increased markedly after co-culture except Bmp2, which was increased gradually when cells were exposed to EMF. Compared to other indices, the expression of Bmp2 in BMSCs was increased sharply in both single-cultured and co-cultured groups when they were exposed to EMF. The mRNA expression of Bmp2 in BMSCs was approximately four times higher in 8-h EMF group than that in the unexposed group. Our results suggest that Bmp2-mediated cellular interaction induced by EMF exposure might play an important role in the osteogenic differentiation of BMSCs.
Similar content being viewed by others
References
Bassett CA, Pilla AA, Pawluk RJ. A non-operative salvage of surgically-resistant pseudarthroses and non-unions by pulsing electromagnetic fields. A preliminary report. Clin Orthop Relat Res, 1977 (124):128–143
Borsalino G, Bagnacani M, Bettati E, et al. Electrical stimulation of human femoral intertrochanteric osteotomies. Double-blind study. Clin Orthop Relat Res, 1988 (237):256–263
Brighton CT, Shaman P, Heppenstall RB, et al. Tibial nonunion treated with direct current, capacitive coupling, or bone graft. Clin Orthop Relat Res, 1995 (321):223–234
Eyres KS, Saleh M, Kanis JA. Effect of pulsed electromagnetic fields on bone formation and bone loss during limb lengthening. Bone, 1996,18(6):505–509
Simmons JW Jr, Mooney V, Thacker I. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electromagnetic fields. Am J Orthop (Belle Mead NJ), 2004,33(1):27–30
Polk C, Postow E. Handbook of biological effects of electromagnetic fields. 2nd ed. Boca Raton, FL: CRC Press, 1996
Fassina L, Visai L, Benazzo F, et al. Effects of electromagnetic stimulation on calcified matrix production by SAOS-2 cells over a polyurethane porous scaffold. Tissue Eng, 2006,12(7):1985–1999
Wang Z, Clark CC, Brighton CT. Up-Regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am, 2006,88(5):1053–1065
Tsai MT, Chang WH, Chang K, et al. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics, 2007,28(7):519–528
Zhang X, Zhang J, Qu X, et al. Effects of different extremely low-frequency electromagnetic fields on osteoblasts. Electromagn Biol Med, 2007,26(3):167–177
Barker AT. Electricity, magnetism and the body: some uses and abuses. J R Soc Health, 1994,114(2):91–97
Leisner S, Shahar R, Aizenberg I, et al. The effect of short-duration, high-intensity electromagnetic pulses on fresh ulnar fractures in rats. J Vet Med A Physiol Pathol Clin Med, 2002,49(1):33–37
Sakai Y, Patterson TE, Ibiwoye MO, et al. Exposure of mouse preosteoblasts to pulsed electromagnetic fields reduces the amount of mature, type I collagen in the extracellular matrix. J Orthop Res, 2006,24(2):242–253
Sollazzo V, Traina GC, DeMattei M, et al. Responses of human MG-63 osteosarcoma cell line and human osteoblast-like cells to pulsed electromagnetic fields. Bioelectromagnetics, 1997,18(8):541–547
Lohmann CH, Schwartz Z, Liu Y, et al. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J Orthop Res, 2000,18(4):637–646
Lohmann CH, Schwartz Z, Liu Y, et al. Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res, 2003,21(2):326–334
Sollazzo V, Traina GC, DeMattei M, et al. Responses of human MG-63 osteosarcoma cell line and human osteoblast-like cells to pulsed electromagnetic fields. Bioelectromagnetics, 1997,18(8):541–547
Yang Y, Tao C, Zhao D, et al. EMF acts on rat bone marrow mesenchymal stem cells to promote differentiation to osteoblasts and to inhibit differentiation to adipocytes. Bioelectromagnetics, 2010,31(4):277–285
Shalhoub V, Conlon D, Tassinari M, et al. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J Cell Biochem, 1992,50(4):425–440
Kwok S, Qin L, Partridge NC, et al. Parathyroid hormone stimulation and PKA signaling of latent transforming growth factor-beta binding protein-1 (LTBP-1) mRNA expression in osteoblastic cells. J Cell Biochem, 2005,95(5):1002–1011
Kaplow LS. Leukocyte alkaline phosphatase cytochemistry: applications and methods. Ann New York Acad Sci, 1969,155(2):911–947
Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem, 1997,64(2):295–312
Chang JK, Li CJ, Wu SC, et al. Effects of anti-inflammatory drugs on proliferation, cytotoxicity and osteogenesis in bone marrow mesenchymal stem cells. Biochemical Pharmacology, 2007,74(9):1371–1382
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 2001,25(4):402–408
Marom R, Shur I, Solomon R, et al. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J Cell Physiol, 2005,202(1):41–48
Wozney JM. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev, 1992,32(2):160–167
Urist MR. Bone morphogenetic protein: the molecularization of skeletal system development. J Bone Miner Res, 1997,12(3):343–346
Marie PJ, Debiais F, Hay E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol Histopathol, 2002,17(3):877–885
Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and cbfa1. Endocr Rev, 2000,21(4):393–411
Guicheux J, Lemonnier J, Ghayor C, et al. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res, 2003,18(11):2060–2068
Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors, 2004,22(4):233–241
Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature, 1997,390(6659):465–471
Nakao A, Imamura T, Souchelnytskyi S, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J, 1997,16(17):5353–5362
Nishimura R, Hata K, Harris SE, et al. Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone, 2002,31(2):303–312
Author information
Authors and Affiliations
Corresponding authors
Additional information
This project was supported by a grant from the National Natural Science Foundation of China (No. 51077065).
Rights and permissions
About this article
Cite this article
Yu, Jz., Wu, H., Yang, Y. et al. Osteogenic differentiation of bone mesenchymal stem cells regulated by osteoblasts under EMF exposure in a co-culture system. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 247–253 (2014). https://doi.org/10.1007/s11596-014-1266-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1266-4