Summary
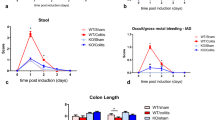
Diabetes patients tend to have the gastrointestinal motility disorder. Although the relationship between the motility disorder and both the neurons and Cajal cells in the enteric nervous system (ENS) is well established, little is known about the role of enteric glial cells (EGCs) in gastric motility in diabetes. This study aimed to examine the expression of the glial marker S100B and morphology of EGCs in gastric tissues and the relationship between activated EGCs and the damage of gastric emptying in diabetic models. The diabetic model of rat was induced with 1% streptozotocin (STZ). The model rats at 7–14 days and at 56–63 days were defined as early diabetic rats and advanced diabetic rats, respectively, and normal rats at the two time periods served as their corresponding controls. The gastric emptying rate of the rats was tested by using the phenol red solution. The ultrastructure of EGCs in the gastric antrum was observed by the transmission electron microscopy, and the expression of S100B in the myenteric plexus was immunohistochemically detected. The results showed that the gastric emptying rate was significantly increased in the early diabetic rats and decreased in the advanced diabetic rats when compared with their corresponding control rats (P<0.01 for both). The ultrastructure of EGCs was mostly normal in both the early diabetic and control groups. Vacuolization of mitochondria and expansion of endoplasmic reticulum occurred in both the advanced diabetic group and its control group, and even the structure of smooth muscle cells and intestinal neurons was destroyed in the advanced diabetic group. The expression level of S100B in the advanced diabetic group was significantly decreased compared with its control group (P<0.05). It was obviously increased in the early diabetic control group when compared with the advanced diabetic control group (P<0.05). However, there was no significant difference in the S100B expression between the early diabetic group and its control group (P>0.05). The findings suggested that the gastric motility dysfunction in diabetes may be associated with the changes of morphology and number of EGCs in the myenteric plexus.
Similar content being viewed by others
References
Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil, 2008,20(Suppl 1):8–19
Gomes P, Chevalier J, Boesmans W, et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil, 2009,21(8):870–e62
Bassotti G, Villanacci V, Fisogni S, et al. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuron-gliopathies. World J Gastroenterol, 2007,13(30):4035–4041
Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil, 2007,19(12):951–960
Braak H, de Vos RA, Bohl J, et al. Gastric alpha-synuclein immunoreactive inclusions in meissner’s and auerbach’s plexuses in cases staged for parkinson’s disease-related brain pathology. Neurosci Lett, 2006,396(1):67–72
Bassotti G, Villanacci V, Cathomas G, et al. Enteric neuropathology of the terminal ileum in patients with intractable slow transit constipation. Hum Pathol, 2006,37(10): 1252–1258
Song GQ, Chen JD. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. J Appl Physiol, 2007,103(5):1560–1564
Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care, 1998,21(4):518–524
Mokdad AH, Ford ES, Bowman BA, et al. The continuing increase of diabetes in the US. Diabetes Care, 2001,24(2):412
Goyal RK, Spiro HM. Gastrointestinal manifestations of diabetes mellitus. Med Clin North Am, 1971,55(4): 1031–1044
Verne GN, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol Clin North Am, 1998,27(4):861–874
Furlan MM, Molinari SL, Miranda Neto MH. Morphoquantitative effects of acute diabetes on the myenteric neurons of the proximal colon of adult rats. Arq Neuropsiquiatr, 2002,60(3-A):576–581
Fregonesi CE, Miranda-Neto MH, Molinari SL, et al. Quantitative study of the myenteric plexus of the stomach of rats with streptozotocin-induced diabetes. Arq Neuropsiquiatr, 2001,5(1):50–53
He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology, 2001,121(2): 427–434
Iantorno G, Bassotti G, Kogan Z, et al. The enteric nervous system in chagasic and idiopathic megacolon. Am J Surg Pathol, 2007,31(3):460–468
Cirillo C, Sarnelli G, Esposito G, et al. S100B protein in the gut: The evidence for enteroglial-sustained intestinal infammation. World J Gastroenterol, 2011,17(10):1261–1266
Ferri GL, Probert L, Cocchia D, et al. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature, 1982,297(5865): 409–412
Ariga H, Imai K, Chen C, et al. Does ghrelin explain accelerated gastric emptying in the early stages of diabetes mellitus? Am J Physiol Regul Integr Comp Physiol, 2008,294(6):R1807–R1812
Takahashi T, Nakamura K, Itoh H, et al. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology, 1997,113(5):1535–1544
Martinez V, Barrachina MD, Wang L, et al. Intracerebroventricular leptin inhibits gastric emptying of a solid nutrient meal in rats. Neuroreport, 1999,10(15):3217–3221
Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol, 2005,58(9):973–977
Bassotti G, Villanacci V, Maurer CA, et al. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut, 2006,55(1):41–46
Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol, 2004,209(1):19–30
Author information
Authors and Affiliations
Corresponding author
Additional information
The project was supported by a grant from the National Nature Science Foundation of China (No. 81170342).
Rights and permissions
About this article
Cite this article
Qi, R., Yang, W. & Chen, J. Role of enteric glial cells in gastric motility in diabetic rats at different stages. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 33, 496–500 (2013). https://doi.org/10.1007/s11596-013-1148-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-013-1148-1