Summary
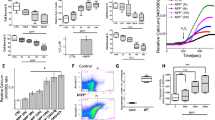
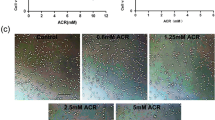
Apoptosis of dopaminergic neurons in the nigrostriatal projection plays a crucial role in the pathogenesis of Parkinson’s disease (PD). Although the detailed mechanisms responsible for dopaminergic neuron loss are still under investigation, oxidative stress is identified as a major contributor for neuronal apoptosis. In the current study, we studied the effects of MPP+, a substrate that mimics oxidative stress, on neuron-like PC12 cells and the underlying mechanisms. PC12 cells were cultured and treated by 100 μmol/L MPP+ for 4, 8, 16, 24 and 48 h, respectively. For drug pretreatment, the PC12 cells were incubated with N-acetyl-l-cysteine (NAC, 5 mmol/L), an antioxidant, SP600125 (20 μmol/L) or PD98059 (100 μmol/L), two pharmacological inhibitors of JNK and ERK1/2, for 1 h before addition of MPP+. Cell apoptosis was measured by flow cytometry. The mRNA expression of Cu2+/Zn2+-SOD, GSH-Px, Bcl-2 and Bax was detected by RT-PCR. The protein expression of p-ERK1/2 and p-JNK was determined by Western blotting. Our results showed that MPP+ exposure could induce substantial PC12 cell apoptosis. The pretreatment of SP600125 or PD98059 could effectively reduce the apoptosis rate by reducing the ratio of Bax/Bcl-2 mRNA levels. MPP+ exposure also induced high level of reactive oxygen species (ROS), marked by dramatic increase of Cu2+/Zn2+-SOD and GSH-Px mRNA levels. The elevated ROS was strongly associated with the activation of JNK and ERK1/2 signal pathways after MPP+ exposure, since the pretreatment of NAC significantly reduced the upregulation of p-JNK and p-ERK1/2. Finally, the pretreatment of SP600125, but not PD98059, alleviated the increase of Cu2+/Zn2+-SOD and GSH-Px mRNAs induced by MPP+, suggesting that the activation of the JNK signal pathway, but not the ERK1/2 signal pathway, could, in some degree, antagonize the generation of ROS induced by oxidative stress. In conclusion, our results suggest that JNK and ERK1/2 signal pathways, which are activated via ROS, play a crucial role in neuronal apoptosis induced by oxidative stress.
Similar content being viewed by others
References
Eldar-finkelman H. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol Med, 2002,8(3):126–132
Beal MF. Mitochondria, oxidative damage and inflammation in Parkinson’s disease. Ann N Y Acad Sci, 2003,991:120–131
Tretter L, Sipos I, Adam-Vizi V. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson’s disease. Neurochem Res, 2004,29(3):569–577
Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol, 2008,4(11):600–609
Felty Q, Xiong WC, Sun D, et al. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry, 2005,44(18):6900–6909
Prabhakar NR, Kumar GK, Nanduri J, et al. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal, 2007,9(9): 1397–1403
Yan Y, Wei CL, Zhang WR, et al. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin, 2006,27(7):821–826
Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta, 2007,1773(8):1213–1226
Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biotechnol, 2005,29(1):57–74
Alexi T, Borlongan CV, Faull RL, et al. Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog Neurobiol, 2000,60(5):409–470
Jellinger KA. Cell death mechanisms in Parkinson’s disease. J Neural Transm, 2000,107(1):1–29
Chen L, Liu L, Luo Y, et al. MAPK and mTOR pathways are involved in cadmium induced neuronal apoptosis. J Neurochem, 2008,105(1):251–261
Behl C. Alzheimer’s disease and oxidative stress: implications for novel therapeutic approaches. Prog Neurobiol, 1999,57(3):301–323
Valencia A, Moran J. Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic Biol Med, 2004,36(9):1112–1125
Chen L, Liu L, Huang S. Cadmium activates MAPK pathway via induction of reactive oxygen species and inhibition of protein phosphatase 2A and 5. Free Radic Biol Med, 2008,45(7):1035–1044
Figueiredo-Pereira ME, Yakushin S, Cohen G. Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells. J Biol Chem, 1998,273(21):12703–12709
Green KN, Peers C. Divergent pathways account for two distinct effects of amyloid beta peptides on exocytosis and Ca2+ currents: involvement of ROS and NFkappaB. J Neurochem, 2002,81(5):1043–1051
Baxter LC, Sparks DL, Johnson SC, et al. Relationship of cognitive measures and gray and white matter in Alzheimer’s disease. J Alzheimers Dis, 2006,9(3):253–260
Lu TH, Hsieh SY, Yen CC, et al. Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicol Lett, 2011,204(1):71–80
Wu J, Mei C, Vlassara H, et al. Oxidative stress-induced JNK activation contributes to proinflammatory phenotype of aging diabetic mesangial cells. Am J Physiol Renal Physiol, 2009,297(6):F1622–F1631
Teng CH, Huang WN, Meng TC. Several dual specificity phosphatases coordinate to control the magnitude and duration of JNK activation in signaling response to oxidative stress. J Biol Chem, 2007,282(39):28 395–28 407
Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta, 2010,1802(4):396–405
Verheij M, Bose R, Lin XH, et al. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature, 1996,380 (6569):75–79
Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 1995,270(5240):1326–1331
Jimenez LA, Zanella C, Fung H, et al. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. J Physiol, 1997,273(5 Pt 1):L1029–L1035
Adler EM, Gough NR, Blundon JA. Differentiation of PC12 cells. Sci STKE, 2006,351:tr9
Wong WW, Puthalakath H. Bcl-2 family proteins: the sentinels of the mitochondrial apoptosis pathway. IUBMB Life, 2008,60(6):390–397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Q., Wang, J., Zhang, Y. et al. Mechanisms of MPP+-induced PC12 cell apoptosis via reactive oxygen species. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 861–866 (2012). https://doi.org/10.1007/s11596-012-1048-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-1048-9