Summary
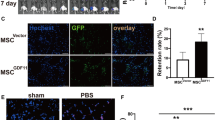
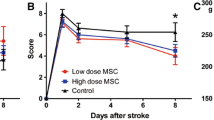
This study explored whether the transplantation of modified marrow stromal cells (MSCs) has angiogenic effects in a left middle cerebral artery occlusion infarction/reperfusion (MCAO I/R) rat model and preliminarily examined the mechanism of angiogenesis following cerebral infarction. MSCs were isolated by using a direct adherent method and cultured. Vascular endothelial growth factor (VEGF) was transfected into MSCs by employing the liposome transfection. The transfection efficiency was measured by the optical density method. The protein expression of VEGF gene before and after transfection was measured by Western blotting. SD rat model of transient occlusion of the left middle cerebral artery was established by using an approach of intra-luminal occlusion. Tetrazolium (TTC) and HE staining were performed to observe the cerebral infarction. ELISAs were used to measure the levels of VEGF in the rat cerebral tissues. The expression patterns of angiopoietin-2 (Ang-2) and CD34 in cells surrounding the area of infarction were immunohistochemistrically oserved. Ang-2 protein expression in the tissue surrounding the area of infarction was measured by Western blotting. VEGF expression in the MSCs increased after transfection at a rate of approximately 28%±3.4%. ELISA showed that the expression of VEGF in the cerebral tissue was significantly increased after induction of infarction, peaking on the 4th day and decreasing to the levels of the sham surgery group (normal) within 7 to 10 days. The VEGF level was significantly higher at each time point in the VEGF-MSC and MSC groups compared to the model group. Moreover, the VEGF level was higher in the VEGF-MSC group than in the MSC group and stayed relatively high until the 10th day. The immunohistochemical results showed that 10 days after the infarction, the number of Ang-2 and CD34-expressing cells in the area surrounding the infarction was significantly higher in the VEGF-MSC group and the MSC group compared to the model group. Moreover, the VEGF level was higher in the VEGF-MSC group than the MSC group. A similar trend in Ang-2 protein expression was revealed by Western blotting. In the MCAO rat model transfected with modified MSCs over-expressing VEGF, compared to the MSC transplantation group, the concentration of VEGF was significantly increased in the brain tissue after cerebral infarction. In addition, the level of Ang-2 was up-regulated, with angiogenesis promoted, the blood supply to the areas surrounding the cerebral infarction increased, and neurological function improved. We are led to speculate that the synergistic effects of VEGF and Ang-2 may be responsible for the angiogenesis following cerebral infarction.
Similar content being viewed by others
References
Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol, 2010,67(4):488–497
Krupinski J, Kaluza J, Wang JM, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke, 1994 25(9):1794–1798
Zhang YK, Han XY, Che ZY, et al. Effects of Buyang Huanwu Tang combined with bone marrow mesenchymal stem cell transplantation on the expression of vegf and ki-67 in the braintissue of the cerebral ischemia-reperfusion model rat. J Traditional Chin Med (Chinese), 2010,30(4): 278–282
Ardelt AA, McCullough LD, Korach KS, et al. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke, 2005,36(2):337–341
Ardelt AA, McCullough LD, Korach KS, et al. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke, 2005,36(2):337–341
Dormady SP, Bashayan O, Dougherty R, et al. Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res, 2001,10(2):125–140
Shichinohe H, Kuroda S, Sugiyama T, et al. Bone marrow stromal cell transplantation attenuates cognitive dysfunction due to chronic cerebral ischemia in rats. Dement Geriatr Cogn Disord, 2010,30(4):293–301
Liu H, Honmou O, Harada K, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006,129(Pt 10):2734–45
Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke, 1994,25:1794–1798
Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review. J Neuroimmune Pharmacol. 2007,2(3):284–289
Zea Longa El, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stoke, 1989:20(1):84–91
Naghdi S, Ansari NN, Mansouri K, et al. The correlation between Modified Ashworth Scale scores and the new index of alpha motoneurones excitability in post-stroke patients. Electromyogr Clin Neurophysiol, 2008,48(2): 109–115
Long L, Li G, Chen W, et al. Distribution of serotonin immunoreactivity in the spiral ganglion neurons of mouse cochlea. Int J Pediatr Otorhinolaryngol, 2008,72(7): 1003–6.
Xu Z, Ford BD. Upregulation of erbB receptors in rat brain after middle cerebral arterial occlusion. Neurosci Lett, 2005,375(3):181–186
Kamimura K, Suda T, Zhang G, et al. Advances in Gene Delivery Systems. Pharmaceut Med, 2011,25(5):293–306
Onda T, Honmou O, Harada K, et al. Therapeutic benefits by human mesenchym al stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab, 2008,28(2):329–340
Hattori Y. Development of non-viral vector for cancer gene therapy. Yakugaku Zasshi, 2010,130(7):917–923
Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J, 2009,4(11): 1559–1572
Wang TZ, Ma AQ, Xu ZY, et al. Differentiation of mesenchymal stem cells into cardiomyocytes induced by cardiomyocytes]. Zhong Nan Da Xue Xue Bao Yi Xue Ban (Chinese), 2005,30(3):270–275
Kazuhiko K, Kimianori N, Takashi T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduced infarct size in the rat middle cerebral artery occlusion model. Molecular Therapy, 2004,9(2):189–197
Valable S, Montaner J, Bellail A, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab, 2005,25(11): 1491–1504
Kolosov A, Aurini L, Williams ED, et al. Intravenous injection of leconotide, an omega conotoxin: synergistic antihyperalgesic effects with morphine in a rat model of bone cancer pain. Pain Med, 2011,12(6):923–941
Wu X, Liu N. The role of Ang/Tie signaling in lymphangiogenesis. Lymphology, 2010,43(2):59–72
Liu XS, Michael Chopp M, Zhang RL, et al. Angiopoietin2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem, 2009,284(34),22680–22689
Heike Beck, Plate KH, Angiogenesis after cerebral ischemia. Acta Neuropathol, 2009,17(5):481–496
Reiss Y. Angiopoietins. Recent Results Cancer Res. 2010,180:3–13
Shin HY, Kim J H, Phi J H, et al. Endogenous neurogenesis and neovascularization in the neocortex of the rat after focal cerebral ischemia. Neuroscience Research, 2008,86(2):356–367
Kademani D, Lewis JT, Lamb DH, et al. Angiogenesis and CD34 expression as a predictor of recurrence in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2009, 67(9):1800–1805
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant from the open fund of Key Laboratory of Molecular Imaging of Hubei Province (No. 2008-72).
Rights and permissions
About this article
Cite this article
Lai, T., Li, M., Zheng, L. et al. Over-expression of VEGF in marrow stromal cells promotes angiogenesis in rats with cerebral infarction via the synergistic effects of VEGF and Ang-2. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 724–731 (2012). https://doi.org/10.1007/s11596-012-1025-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-1025-3