Summary
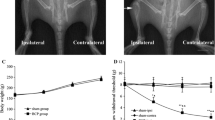
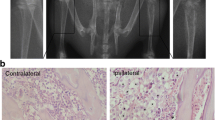
Descending nociceptive modulation from the supraspinal structures plays an important role in cancer-induced bone pain (CIBP). Rostral ventromedial medulla (RVM) is a critical component of descending nociceptive facilitation circuitry, but so far the mechanisms are poorly known. In this study, we investigated the role of RVM glial activation in the descending nociceptive facilitation circuitry in a CIBP rat model. CIBP rats showed significant activation of microglia and astrocytes, and also up-regulation of phosphorylated p38 mitogen-activated protein kinase (p38 MAPK) and pro-inflammatory mediators released by glial cells (IL-1β, IL-6, TNF-α and brain-derived neurotrophic factor) in the RVM. Stereotaxic microinjection of the glial inhibitors (minocycline and fluorocitrate) into CIBP rats’ RVM could reverse the glial activation and significantly attenuate mechanical allodynia in a time-dependent manner. RVM microinjection of p38 MAPK inhibitor (SB203580) abolished the activation of microglia, reversed the associated up-regulation of pro-inflammatory mediators and significantly attenuated mechanical allodynia. Taken together, these results suggest that RVM glial activation is involved in the pathogenesis of CIBP. RVM microglial p38 MAPK signaling pathway is activated and leads to the release of downstream pro-inflammatory mediators, which contribute to the descending facilitation of CIBP.
Similar content being viewed by others
References
Colvin L, Fallon M. Challenges in cancer pain management—bone pain. Eur J Cancer, 2008,44(8):1083–1090
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer, 2011,11(6) 411–425
Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al. Bone cancer pain. Ann N Y Acad Sci, 2010,1198: 173–181
Goblirsch MJ, Zwolak PP, Clohisy DR. Biology of bone cancer pain. Clin Cancer Res, 2006,12(20):6231s–6235s
Millan MJ. Descending control of pain. Prog Neurobiol, 2002,66(6):355–474
Vera-Portocarrero LP, Zhang ET, Ossipov MH, et al. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience, 2006,140(4):1311–1320
Porreca F, Burgess SE, Gardell LR, et al. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci, 2001,21(14):5281–5288
Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest, 2010,120(11):3779–3787
Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci, 2002, 25(6):319–325
Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci, 2009,30(2):229–241
Wei F, Guo W, Zou S, et al. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci, 2008,28(42):10 482–10 495
Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain, 2007,3:33
Kohno T. Neuropathic pain and neuron-glia interactions in the spinal cord. J Anesth, 2010,24(2):325–327
Geis C, Graulich M, Wissmann A, et al. Evoked pain behavior and spinal glia activation is dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer pain. Neuroscience, 2010,169(1):463–474
Zhang RX, Liu B, Wang L, et al. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain, 2005,118(1–2): 125–136
Cao F, Gao F, Xu AJ, et al. Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res, 2010, 1326:162–173
Laalou FZ, de Vasconcelos AP, Oberling P, et al. Involvement of the basal cholinergic forebrain in the mediation of general (propofol) anesthesia. Anesthesiology, 2008,108(5):888–896
Wei F, Dubner R, Zou S, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci, 2010,30(25):8624–8636
Fox A, Medhurst S, Courade JP, et al. Anti-hyperalgesic activity of the cox-2 inhibitor lumiracoxib in a model of bone cancer pain in the rat. Pain, 2004,107(1–2):33–40
Wang Y, Li X, Cao L, et al. Analgesic effect of diprospan in rats with trigeminal neuralgia. J Huazhong Univ Sci Technolog [Med Sci], 2011,31(3):395–399
Cao F, Chen SS, Yan XF, et al. Evaluation of side effects through selective ablation of the mu opioid receptor expressing descending nociceptive facilitatory neurons in the rostral ventromedial medulla with dermorphin-saporin. Neurotoxicology, 2009,30(6):1096–1106
Wang GM, Tian XB, Chen JP, et al. Prevention of neuropathic pain in an animal model of spare nerve injury following oral immunization with recombinant adenovirus serotype 5-mediated NR2B gene transfer. Gene Ther, 2007,14(24):1681–1687
Shimizu K, Guo W, Wang H, et al. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain, 2009,5:75
Hosoi R, Okada M, Hatazawa J, et al. Effect of astrocytic energy metabolism depressant on 14C-acetate uptake in intact rat brain. J Cereb Blood Flow Metab, 2004,24(2): 188–190
Romero-Sandoval A, Chai N, Nutile-McMenemy N, et al. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res, 2008, 1219:116–126
Mao-Ying QL, Zhao J, Dong ZQ, et al. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun, 2006,345(4):1292–1298
Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci, 2007,10(11): 1361–1368
Storkson RV, Kjorsvik A, Tjolsen A, et al. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods, 1996,65(2):167–172
Kristensen JD, Post C, Gordh T Jr, et al. Spinal cord morphology and antinociception after chronic intrathecal administration of excitatory amino acid antagonists in the rat. Pain, 1993,54(3):309–316
Tsang BK, He Z, Ma T, et al. Decreased paralysis and better motor coordination with microspinal versus PE10 intrathecal catheters in pain study rats. Anesth Analg, 1997,84(3):591–594
Author information
Authors and Affiliations
Corresponding authors
Additional information
This project was supported by grants from the National Natural Science Foundation of China (No. 30901396, No. 81070890, No. 30872441 and No. 81171259).
Rights and permissions
About this article
Cite this article
Liu, X., Bu, H., Liu, C. et al. Inhibition of glial activation in rostral ventromedial medulla attenuates mechanical allodynia in a rat model of cancer-induced bone pain. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 291–298 (2012). https://doi.org/10.1007/s11596-012-0051-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-0051-5