Summary
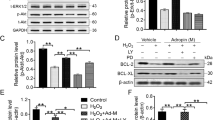
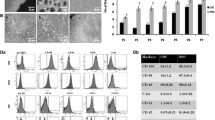
Bone marrow mesenchymal stem cells (MSCs) have shown potential for cardiac repair following myocardial injury, but this approach is limited by their poor viability after transplantation. The present study was to investigate whether trimetazidine (TMZ) could improve survival of MSCs in an ex vitro model of hypoxia, as well as survival, differentiation, and subsequent activities of transplanted MSCs in rat hearts with acute myocardial infarction (AMI). MSCs at passage 3 were examined for their viability and apoptosis under a transmission electron microscope, and by using flow cytometry following culture in serum-free medium and exposure to hypoxia (5% CO2, 95% N2) for 12 h with or without TMZ. Thirty Wistar rats were divided into 3 groups (n=10 each group), including group I (AMI control), group II (MSCs transplantation alone), and group III (TMZ+MSCs). Rat MSCs (4×107) were injected into peri-infarct myocardium (MSCs group and TMZ+MSCs group) 30 min after coronary artery ligation. The rats in TMZ+MSCs group were additionally fed on TMZ (2.08 mg·kg−1·day−1) from day 3 before AMI to day 28 after AMI. Cardiac structure and function were assessed by echocardiography at 28th day after transplantation. Blood samples were collected before the start of TMZ therapy (baseline), and 24 and 48 h after AMI, and inflammatory cytokines (CRP, TNF-α) were measured. Then the survival and differentiation of transplanted cells in vivo were detected by immunofluorescent staining. The cellular apoptosis in the peri-infarct region was detected by using TUNEL assay. Furthermore, apoptosis-related proteins (Bcl-2, Bax) within the post-infarcted myocardium were detected by using Western blotting. In hypoxic culture, the TMZ-treated MSCs displayed a two-fold decrease in apoptosis under serum-free medium and hypoxia environment. In vivo, cardiac infarct size was significantly reduced, and cardiac function significantly improved in MSCs and TMZ+MSCs groups as compared with those in the AMI control group. Combined treatment of TMZ with MSCs implantation demonstrated further decreased MSCs apoptosis, further increased MSCs viability, further decreased infarct size, and further improved cardiac function as compared with MSCs alone. The baseline levels of inflammatory cytokines (CRP, TNF-α) had no significant difference among the groups. In contrast, all parameters at 24 h were lower in TMZ+MSCs group than those in MSCs group. Furthermore, Western blotting indicated that the expression of anti-apoptotic protein Bcl-2 was up-regulated, while the pro-apoptotic protein Bax was down-regulated in the TMZ+MSCs group, compared with that in the MSCs group. It is suggested that implantation of MSCs combined with TMZ treatment is superior to MSCs monotherapy for MSCs viability and cardiac function recovery.
Similar content being viewed by others
References
Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation, 1993,88(1):107–115
Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest, 1999,103(5):697–705
Song H, Kwon K, Lim S, et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells, 2005,19(3):402–407
Bianchi G, Banfi A, Mastrogiacomo M, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res, 2003,287(1):98–105
Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med, 2003,9(9): 1195–1201
Maridonneau PI, Harpey C. Effects of trimetazidine on membrane damage reduced by oxygen free radicals in human red cells. Br J Clin Pharmacol, 1985,20(2): 148–151
Maupoil V, Rochette L, Tabard A, et al. Direct measurement of free radical generation in isolated rat heart by electron paramagnetic resonance spectroscopy: effect of trimetazidine. Adv Exp Med Biol, 1990,264:373–376
Reymond F, Steyaert G, Carrupt PA, et al. The pH-partition profile of the anti-ischemic drug trimetazidine may explain its reduction of intracellular acidosis. Pharm Res, 1999,16(5):616–624
Renaud JF. Internal pH, Na+, and Ca2+ regulation by trimetazidine during cardiac cell acidosis. Cardiovasc Drugs Ther, 1988,1(6):677–686
Salducci MD, Chauvet-Monges AM, Tillement JP, et al. Trimetazidine reverses calcium accumulation and impairment of phosphorylation induced by cyclosporine A in isolated rat liver mitochondria. J Pharmacol Exp Ther, 1996,277(1):417–422
Wang JS, Shum-Tim D, Galipeau J, et al. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg, 2000,120(5):999–1006
Suzuki K, Smolenski RT, Jayakumar J, et al. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation, 2000,102(19 Suppl 3):III216–221
Jiang S, Haider HKh, Idris NM, et al. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res, 2006,99(7): 776–784
Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J, 2006,20(6):661–669
Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells, 2007,25(8):2118–2127
Bartunek J, Croissant JD, Wijins W, et al. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol, 2007,292(2):H1095–1104
Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol, 2005,46(7):1339–1350
Jo JI, Nagaya N, Miyahara Y, et al. Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial infarction: benefit of a novel nonviral vector, Cationized Dextran. Tissue Eng, 2007,13(2):313–322
Noble MI, Belcher PR, Drake-Holland AJ. Limitation of infarct size by trimetazidine in the rabbit. Am J Cardiol, 1995,76(6):41B–44B
Di Pasquale P, Lo Verso P, Bucca V, et al. Effects of trimetazidine administration before thrombolysis in patients with anterior myocardial infarction: short-term and long-term results. Cardiovasc Drugs Ther, 1999,13(5): 423–428
Freude B, Masters TN, Robicsek F, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol, 2000,32(2):197–208
Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science, 1995,267(5203):1456–1462
Entman ML, Michael L, Rossen RD, et al. Inflammation in the course of early myocardial ischemia. FASEB J, 1991,5(11):2529–2537
Kaminski KA, Bonda TA, Korecki J, et al. Oxidative stress and neutrophil activation-the two keystones of ischemia/reperfusion injury. Int J Cardiol, 2002,86(1): 41–59
Tokuda H, Kozawa O, Uematsu T. Basic fibroblast growth factor stimulates vascular endothelial growth factor release in osteoblasts: divergent regulation by p42/p44 mitogen-activated protein kinase and p38 mitogen-activated protein kinase. J Bone Miner Res, 2000, 15(12):2371–2379
Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol, 2000,32(2):115–120
Debiais F, Lefevre G, Lemonnier J, et al. Fibroblast growth factor-2 induces osteoblast survival through a phosphatidylinositol 3-kinase-dependent-beta-catenin-independent signaling pathway. Exp Cell Res, 2004,297(1)235–246
Lopaschuk GD. Optimising cardiac energy metabolism: how can fatty acid and carbohydrate metabolism be manipulated? Coronary Artery Dis, 2001,12(Suppl 1):8–11
Author information
Authors and Affiliations
Corresponding author
Additional information
Both authors contributed equally to this work.
This project was supported by grants from the National Natural Science Foundation of China (No. 30700314) and Wuhan Science and Technology Bureau of Hubei province, China (No. 20065004116-02).
Rights and permissions
About this article
Cite this article
Xu, H., Zhu, G. & Tian, Y. Protective effects of trimetazidine on bone marrow mesenchymal stem cells viability in an ex vivo model of hypoxia and in vivo model of locally myocardial ischemia. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 36–41 (2012). https://doi.org/10.1007/s11596-012-0006-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-0006-x