Summary
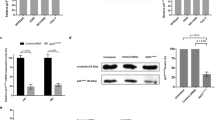
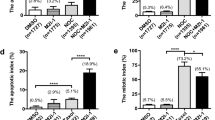
In this article, the status of spindle assembly checkpoint and the alteration of its major component, Mad2 protein level were examined in A2780 and SKOV3 ovarian cancer cell lines. Recombinant eukaryotic expression plasmid pEGFP-Mad2 was transfected into paclitaxel-resistant SKOV3 cells and Mad2 protein was knocked down by Mad2-specific siRNA in paclitaxel-sensitive A2780 cells. Then the expression level of Mad2 gene was detected by Western blotting. Flow cytometry revealed that SKOV3 cells were not fully arrested in G2/M phase in contrast to A2780 cells in the presence of paclitaxel. However, paclitaxel sensitivity assay showed that sensitivity to paclitaxel was reversed after the transfection in both cell lines in terms of number of cells arrested at G2/M phase and the expression of Bcl-2 was significantly changed. These results suggest that weakened spindle checkpoint with reduced expression of Mad2 is associated with resistance to paclitaxel in ovarian cells and Bcl-2 may be involved in this process.
Similar content being viewed by others
References
Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med, 1997,48:353–374
Kovár J, Ehrlichová M, Smejkalová B, et al. Comparison of cell death-inducing effect of novel taxane SB-T-1216 and paclitaxel in breast cancer cells. Anticancer Res, 2009, 29(8):2951–2960
Rowinsky EK, Donehower RC. Paclitaxel. New Engl J Med, 1995,332(15):1004–1014
Winey E, Huneycutt BJ. Centrosomes and checkpoints: the MPS1 family of kinases. Oncogene, 2002,21(40): 6161–6169
Fisk FA, Winey M. Mps1 flies into new areas. Curr Biol, 2004,14(24):R1058–R1060
Zhou J, Yao J, Joshi HC. Attachment and tension in the spindle assembly checkpoint. J Cell Sci, 2002,115(18): 3547–3555
Burke DJ, Stukenberg PT. Linking kinetochore-microtubule binding to the spindle checkpoint. Dev Cell, 2008,14(4):474–479
Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review). Int J Cancer, 1999, 83(2):151–156
Singh P, Rathinasamy K, Mohan R, et al. Microtubule assembly dynamics: an attractive target for anticancer drugs. IUBMB Life, 2008,60(6):368–375
Takahashi T, Haruki N, Nomoto S. Identification of frequent impairment of the mitotic checkpoint and molecular analysis of the mitotic checkpoint genes, hsMAD2 and p55CDC in human lung cancers. Oncogene, 1999,18(30):4295–4300
Wang X, Jin DY, Wong YC, et al. Correlation of defective mitotic checkpoint with aberrantly reduced expression of MAD2 protein in nasopharyngeal carcinoma cells. Carcinogenesis, 2000,21(12):2293–2297
Wang X, Jin DY, Ng RW, et al. Significance of MAD2 expression to mitotic checkpoint control in ovarian cancer cells. Cancer Res, 2002,62(6):1662–1668
McGrogan BT, Gilmartin B, Carney DN, et al. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta, 2008,1785(2):96–132
González Martín A. Anti-angiogenic therapy in ovarian cancer: a great expectation to be confirmed. Clin Transl Oncol, 2009,11(9):559–560
Jemal A, Murray T, Samuels A, et al. Cancer statistics. CA Cancer J Clin, 2003,53(1):5–26
Richardson A, Kaye SB. Drug resistance in ovarian cancer: the emerging importance of gene transcription and spatio-temporal regulation of resistance. Drug Resist Updat, 2005,8(5):311–321
Yamada HY, Gorbsky GJ. Spindle checkpoint function and cellular sensitivity to antimitotic drugs. Mol Cancer Ther, 2006,5(12):2963–2969
Malmanche N, Maia A, Claudio ES. The spindle assembly checkpoint: Preventing chromosome mis-segregation during mitosis and meiosis. FEBS Letters, 2006,580(12): 2888–2895
Zhou T, Bao Y, Ye S, et al. Effect of spindle checkpoint on Akt2-mediated paclitaxel-resistance in A2780 ovarian cancer cells. J Huazhong Univ Sci Technolog [Med Sci], 2010,30(2):206–11
Gorbsky GJ, Chen RH, Murray AW. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J Cell Biol, 1998,141(5): 1193–1205
Sudo T, Nitta M, Saya H, et al. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res, 2004,64(7):2502–2508
Lee EA, Keutmann MK, Dowling ML, et al. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther, 2004,3(6):661–669
Srivastava RK, Mi QS, Hardwick JM, et al. Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Proc Natl Acad Sci USA, 1999, 96(7):3775–80
Wolter KG, Wang SJ, Henson BS, et al. (−)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia, 2006,8(3):163–172
Mackler NJ, Pienta KJ. Drug insight: Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol, 2005, 2(2):92–100
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a Joint Research Fund for Young Scholars Abroad (No. 30528012), National Key Basic Research Program Foundation of China (Program 973) (No. 2009CB521800) and Key Project of Chinese Ministry of Education (No. 108089).
Rights and permissions
About this article
Cite this article
Hao, X., Zhou, Z., Ye, S. et al. Effect of Mad2 on paclitaxel-induced cell death in ovarian cancer cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 30, 620–625 (2010). https://doi.org/10.1007/s11596-010-0553-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-010-0553-y