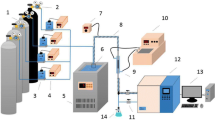
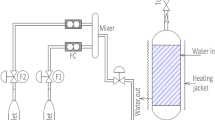
Abstract
Walnut-shell activated carbons (WSACs) were prepared by the KOH chemical activation. The effects of carbonization temperature, activation temperature, and ratio of KOH to chars on the pore development of WSACs were investigated. Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), and scanning electron microscopy (SEM) were employed to characterize the microstructure and morphology of WSACs. Methanol adsorption performance onto the optimal WSAC and the coal-based AC were also investigated. The results show that the optimal preparation conditions are a carbonization temperature of 700 °C, an activation temperature of 700 °C, and a mass ratio of 3. The BET surface area, the micropore volume, and the micropore volume percentage of the optimal WASC are 1636 m2/g, 0.641 cm3/g and 81.97%, respectively. There are a lot of micropores and a certain amount of meso- and macropores. The characteristics of the amorphous state are identified. The results show that the optimal WSAC is favorable for methanol adsorption. The equilibrium adsorption capacity of the optimal WSAC is 248.02mg/g. It is shown that the equilibrium adsorption capacity of the optimal WSAC is almost equivalent to that of the common activated carbon. Therefore the optimal WSAC could be a potential adsorbent for the solar energy adsorption refrigeration cycle.
Similar content being viewed by others
References
Wang RZ, Wu JY, Dai YJ. Adsorption Refrigeration[M]. Beijing: China Machine Press; 2002(in Chinese)
Wang RZ. Adsorption Refrigeration Research in Shanghai Jiao Tong University[J]. Renew. Sust. Energ. Rev., 2001, 5(1): 1–37
Wang RZ, Oliveira RG. Adsorption Refrigeration-An Efficient Way to Make Good Use of Waste Heat and Solar Energy[J]. Prog. Energ. Combust., 2006, 32(4): 424–458
Wang LW, Wang RZ, Oliveira RG. A review on Adsorption Working Pairs for Refrigeration[J]. Renew. Sust. Energ. Rev., 2009, 13(3): 518–534
Ji X, Li M, Fan JQ, et al. Structure Optimization and Performance Experiments of a Solar-powered Finned-tube Adsorption Refrigeration System[J]. Appl. Energ., 2014, 113: 1293–1300
Abdul Majeed Am, Suliman My, Sopian K. Weather Effect on the Solar Adsorption Air-conditioning System using Activated Carbon Fiber/Ethanol as Pair of Refrigeration: A Case Study of Malaysia[J]. Res. J. Appl. Sci. Eng. Tech., 2014, 7(5): 1069–1075
Li M, Huang HB, Wang RZ, et al. Experimental Study on Adsorbent of Activated Carbon with Refrigerant of Methanol and Ethanol for Solar Ice Maker[J]. Renew. Energ., 2004, 29(15): 2235–2244
Shmroukh AN, Ali AHH, Abel-Rahman AK. Experimental Study on Adsorption Capacity of Activated Carbon Pairs with Different Refrigerants[J]. International Journal of Chemical, Materials Science and Engineering, 2013, 7(11): 38–44
Zhao HZ, ZHANG Min, LIU Zhen-yan, et al. Mechanical and Experimental Study on Freeze Proof Solar Powered Adsorption Cooling Tube Using Active Carbon/Methanol Working Pair[J]. Energ. Convers. Manage., 2008, 49(8): 2434–2438
Dong YB, Lin H, Liu QL, et al. Treatment of Flotation Wastewater Using Biological Activated Carbon[J]. J. Cent. South. Univ., 2014, 21(9): 3580–3587
Yu QF, Li M, Ning P, et al. Preparation and Phosphine Adsorption of Activated Carbon Prepared from Walnut Shells by KOH Chemical Activation[J]. Sep. Sci. Technol., 2014, 49(15): 2366–2375
Food and Agriculture Organization of the United Nation. The Statistical Data about the Walnut Production of China in 2012[DB/OL]. http://faostat3. fao. org/home/E, 2013-03-05
Sun K, Jiang JC. Preparation and Characterization of Activated Carbon from Rubber-seed Shell by Physical Activation with Steam[J]. Biomass. Bioenerg., 2010, 34(4): 539–544
Okada K, Yamamoto N, Kameshima Y, et al. Adsorption Properties of Activated Carbon from Waste Newspaper Prepared by Chemical and Physical Activation[J]. J. Colloid. Interf. Sci., 2003, 262(1): 194–199
Yang J, Qiu KQ. Preparation of Activated Carbon by Chemical Activation under Vacuum[J]. Environ. Sci. Technol., 2009, 43(9): 3385–3390
Li X, Wang GZ, Li WG, et al. Adsorption of Acid and Basic Dyes by Sludge-based Activated Carbon: Isotherm and Kinetic Studies[J]. J. Cent. South. Univ., 2015, 22(1): 103–113
Lozano-Castello D, Lillo-Rodenas MA, Cazorla-Amoros D, et al. Preparation of Activated Carbons from Spanish Anthracite I. Activation by KOH[J]. Carbon, 2001, 39(5): 741–749
Ichcho S, Khouya E, Fakhi S, et al. Influence of the Experimental Conditions on Porosity and Structure of Adsorbents Elaborated from Moroccan Oil Shale of Timahdit by Chemical Activation[J]. J. Hazard. Mater., 2005, 118(1-3): 45–51
Tsai WT, Chang CY, Lee SL. A Low Cost Adsorbent from Agricultural Waste Corn Cob by Zinc Chloride Activation[J]. Bioresource. Technol., 1998, 64(3): 211–217
D5832-98. Standard test Method for Volatile Matter Content of Activated Carbon Samples[S]. ASTM International, West Conshohocken, PA, www.astm.org, 1998
D2866-99. Standard Test Method for Total Ash Content of Activated Carbon[S]. ASTM International, West Conshohocken, PA, www. astm. org, 1999
Guo J, Lua AC. Microporous Activated Carbons Prepared from Palm Shell by Thermal Activation and Their Application to Sulfur Dioxide Adsorption[J]. J. Colloid. Interf. Sci., 2002, 251(2): 242–247
Ioannidou O, Zabaniotou A. Agricultural Residues as Precursors for Activated Carbon Production-A Review[J]. Renew. Sust. Energ. Rev., 2007, 11(9): 1966–2005
Lua AC, Yang T. Characteristics of Activated Carbon Prepared from Pistachio-nut Shell by Zinc Chloride Activation under Nitrogen and Vacuum Conditions[J]. J. Colloid. Interf. Sci., 2005, 290(2): 505–513
Yang T, Lua AC. Characteristics of Activated Carbons Prepared from Pistachio-nut Shells by Potassium Hydroxide Activation[J]. Micropor. Mesopor. Mat., 2003, 63(1-3): 113–124
Lua AC, Yang T. Effect of Activation Temperature on the Textural and Chemical Properties of Potassium Hydroxide Activated Carbon Prepared from Pistachio-nut Shell[J]. J. Colloid. Interf. Sci., 2004, 274(2): 594–601
Liu QS, Zheng T, Wang P, et al. Preparation and Characterization of Activated Carbon from Bamboo by Microwave-induced Phosphoric Acid Activation[J]. Ind. Crop. Prod., 2010, 31(2): 233–238
Sun Y, Webley PA. Preparation of Activated Carbons from Corncob with Large Specific Surface Area by a Variety of Chemical Activators and Their Application in Gas Storage[J]. Chem. Eng. J., 2010, 162(3): 883–892
Yang Jn, Qiu KQ. Preparation of Activated Carbons from Walnut Shells Via Vacuum Chemical Activation and Their Application for Methylene Blue Removal[J]. Chem. Eng. J., 2010, 165(1): 209–217
Lua AC, Yang T. Effects of Vacuum Pyrolysis Conditions on the Characteristics of Activated Carbons Derived from Pistachio-nut Shells [J]. J. Colloid. Interf. Sci., 2004, 276(2): 364–372
Chen XR, Huang B, Jiang MS. Study on the Charring Process of Chinese Fir Thinning Wood under Different Conditions by Comparisons of FTIR[J]. Chemical Industry and Engineering Progress, 2008, 27(3): 429–434, 39(in Chinese)
Yang T, Lua AC. Textural and Chemical Properties of Zinc Chloride Activated Carbons Prepared from Pistachio-nut Shells[J]. Mater. Chem. Phys., 2006, 100(2-3): 438–444
Stephan AM, Kumar TP, Thomas S, et al. Pyrolitic Carbon from Biomass Precursors as Anode Materials for Lithium Batteries[J]. Mat. Sci. Eng. A-Struct., 2006, 430(1-2): 132–137
Hwang YJ, Jeong SK, Nahm KS, et al. Pyrolytic Carbon Derived from Coffee Shells as Anode Materials for Lithium Batteries[J]. J. Phys. Chem. Solids., 2007, 68(2): 182–188
Passos E, Meunier F, Gianola JC. Thermodynamic Performance Improvement of an Intermittent Solar-powered Refrigeration Cycle Using Adsorption of Methanol on Activated Carbon[J]. Journal of Heat Recovery Systems, 1986, 6(3): 259–264
Wang RZ, Jia JP, Teng Y, et al. A Potential Solid Adsorption Refrigeration Pair-Active Carbon Fiber-Methanol[J]. Acta Energiae Solaris Sinica, 1997, 18(2): 222–227(in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (Nos. U1137605, 51366014, 51466017, and 51566017), the General Program of Yunnan Provincial Applied Fundamental Research (No. 2011FZ076), and the Scientific Research Training Foundation of Undergraduate (No. ky2014-179)
Rights and permissions
About this article
Cite this article
Yu, Q., Li, M., Ji, X. et al. Characterization and methanol adsorption of walnut-shell activated carbon prepared by KOH activation. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 31, 260–268 (2016). https://doi.org/10.1007/s11595-016-1362-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-016-1362-3