Abstract
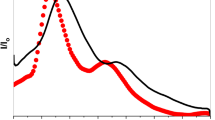
Tin was found in the bottom of float borosilicate glasses. To simulate the enriched amounts of SnO found on the surface of the float borosilicate glasses, a series of glasses were produced in which the stannous concentration was varied from 0.1 wt% to 9.0 wt%, while the relative concentration of other components were held constant. Infrared spectra were obtained to probe the effect of increased amounts of SnO on the structure of the glass samples. The results show that SnO plays the role of an intermediate in glasses studied. When FO/SnO>1.0, SnO takes the role of network-former. And when FO/SnO<1.0, SnO can give the free oxygen as network-modifier. Besides, SnO has intensive effect on thermal performance of borosilicate glasses.
Similar content being viewed by others
References
J F Bent, A C Hannon, D Holland, et al. The Structure of Tin Silicate Glasses[J]. J. Non-Cryst. Solids, 1998, 232–234:300–308
K F E Williams, C E Johnson. Mossbauer Spectra of Tin in Binary Si-Sn Oxide Glasses[J]. J. Phys.:Condens. Matter, 1995, 7:9485–9498
E I Kamitsos, A P Patsis, M A Karakassides, et al. Infrared Reflectance Spectra of Lithium Borate Glasses[J]. J. Non-Cryst. Solids, 1990, 126: 52–67
A H Verhofe, H W Den-Hartog. Infrared Spectroscopy of Network and Cation Dynamics in Binary and Mixed Alkali Borate Glasses[J]. J. Non-Cryst. Solids, 1995, 182:221–234
E I Kamitsos, M A Karakassides, G D Chyssikos. Vibrational Spectra of Magnesium-Sodium-Borate Glasses. 2. Raman and Mid-Infrared Investigation of the Network Structure[J]. J. Phys. Chem., 1987, 91(5):1073–1079
E I Kamitsos, M A Karakassides, G D Chyssikos. A Vibrational Study of Lithium Borate Glasses with High Li2O Conten[J]. Phys. Chem. Glasses, 1987, 28:203–209
J Krogh-Moe. Interpretation of the Infra-red Spectra of Boron Oxide and Alkali Borate Glasses[J]. Phys. Chem. Glasses, 1965, 6:46–54
J Wong. In Borate Glasses: Structure, Applications[M]. New York: Plenum Press, 1977:297
YI Jialiang. Further Studies on the IR Spectra of Silicate Glasses[J]. J. Non-Cryst. Solids, 1986, 84:114–119
J G Wood, S Prabakar, Karl T Mueller, et al. The Effects of Antimony Oxide on the Structure of Alkaline-earth Alumino Borosilicate Glasses[J]. J. Non-Cryst. Solids, 2004, 349:276–284
G El-Damrawi, W Muller-Warmuth, H Doweidar, et al. 11B, 29Si and 27Al Nuclear Magnetic Resonance Studies of Na2O-Al2O3-B2O3-SiO2 Glasses[J]. Phys. Chem. Glasses, 1993, 34:52–57
W Vogel. Glass Chemistry[M]. Second Ed.. Berlin: Springer-Verlag, 1994:144–145
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the Key Technologies Program from Department of Science and Technology of Hubei Province(No.2004AA1031303)
Rights and permissions
About this article
Cite this article
Lu, P., Cheng, J. & Wan, J. Effects of SnO on structure and properties of borosilicate glasses. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 23, 547–550 (2008). https://doi.org/10.1007/s11595-006-4547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-006-4547-3