Abstract
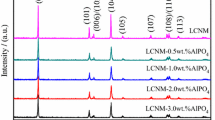
A simple wet chemical method was used to coat a layer of LaF3 on the surface of LiNi0.5Co0.2Mn0.3O2(NCM523) to improve the electrochemical performance. Through the characterization of X-ray diffractometer (XRD), scanning electron microscope (SEM), X-ray energy dispersive spectrometer (EDS), and X-ray photoelectron spectroscopy (XPS), it can be seen that the LaF3 coating can be uniformly coated on the surface of the material and will not change the crystal structure and micro-morphology of the material. In this work, the amount of LaF3 coating on the materials is 0.0 wt%, 0.5 wt%, 1.0 wt%, and 2.0 wt%, respectively. Under the condition of the optimum coating amount of 1 wt%, the rate performance and cycle performance of the coated material can be improved obviously. After 100 cycles at a high cut-off voltage of 4.6 V, the capacity retention of the 1 wt% LaF3 coated material is 88.7%, which is higher than 80.4% of the bare material. And through EIS analysis, the coated material after 50 cycles still has excellent lithium ion diffusion kinetics. This may be due to the fact that an appropriate amount of LaF3 coating can effectively avoid direct contact between cathode materials and electrolytes, inhibit the generation of oxygen vacancies, and reduce unnecessary side effects. This could provide a new idea for improving the electrochemical performance of LiNi0.5Co0.2Mn0.3O2 at high cut-off voltage.









Similar content being viewed by others
References
Li M, Lu J, Chen Z, Amine K (2018) 30 years of lithium-ion batteries. Adv Mater 1800561:1–24
Zhou H, Xin F, Pei B, Whittingham MS (2019) What limits the capacity of layered oxide cathodes in lithium batteries? ACS Energy Lett 4:1902–1906
Zha Q, Hu N, Song C, Hou H, Liao S, Zha G (2021) Improving cycle stability of Ni-rich LiNi0.8Mn0.1Co0.1O2 cathode materials by Li4Ti5O12 coating. Ionics 28:1047–1054
Krishna KS, Ghosh S, Ghosal P, Martha SK (2017) Synergistic effect of 3D electrode architecture and fluorine doping of Li1.2Ni0.15Mn0.55Co0.1O2 for high energy density lithium-ion batteries. J Power Sources 356:115–123
Song L, Li X, Xiao Z, Li L, Cao Z, Zhu H (2018) Effect of Zr doping and Li2O-2B2O3 layer on the structural electrochemical properties of LiNi0.5Co0.2Mn0.3O2 cathode material: experiments and first-principle calculations. Ionics 25:2017–2026
Zhang Z, Qiu J, Yu M, Jin C, Yang B, Guo G (2020) Performance of Al-doped LiNi1/3Co1/3Mn1/3O2 synthesized from spent lithium ion batteries by sol-gel method. Vacuum 172
Choi NS, Han J-G, Ha SY, Park I, Back CK (2015) Recent advances in the electrolytes for interfacial stability of high-voltage cathodes in lithium-ion batteries. RSC Adv 5:2732–2748
Bak SM, Hu E, Zhou Y, Yu X, Senanayake SD, Cho SJ, Kim KB, Chung KY, Yang XQ, Nam KW (2014) Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl Mater Interfaces 6:22594–22601
Liu BS, Zhang SH, Yu YG, Liu JH, He X, Sun ZJ, Yu ZQ, Wu YM, Wang ZB (2021) Interface crystal domain regulation via TiO2 surface modification enhancing stability of layered LiNi0.5Co0.2Mn0.3O2 for lithium-ion batteries. Ionics 27:1871–1880
Zhang H, Zhang Y, Xu T, John AE, Li Y, Li W, Zhu B (2016) Poly(m-phenylene isophthalamide) separator for improving the heat resistance and power density of lithium-ion batteries. J Power Sources 329:8–6
He M, Su CC, Peebles C, Feng Z, Connell JG, Liao C, Wang Y, Shkrob IA, Zhang Z (2016) Mechanistic insight in the function of phosphite additives for protection of LiNi0.5Co0.2Mn0.3O2 cathode in high voltage Li-ion cells. ACS Appl Mater Interfaces 8:11450–11458
Liu T, Zhao SX, Wang KZ, Gou LL, Nan CW (2015) Improved rate capability and cycle stability of Li[Ni0.5Co0.2Mn0.3]O2 with Li2MnO3 coating under high cut-off voltage. Appl Surf Sci 355:1222–1228
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed Engl 54:4440–4457
Li GY, Zhang ZJ, Wang R, Hung ZL, Zuo ZC, Zhou HH (2016) Effect of trace Al surface doping on the structure, surface chemistry and low temperature performance of LiNi0.5Co0.2Mn0.3O2 cathode. Electrochim Acta 212:399–407
Li Y, Liu X, Ren D, Hsu H, Xu GL, Hou J, Wang L, Feng X, Lu L, Xu W, Ren Y, Li R, He X, Amine K, Ouyang M (2020) Toward a high-voltage fast-charging pouch cell with TiO2 cathode coating and enhanced battery safety. Nano Energy 71:104643
Yuan M, Li Y, Chen Q, Chen C, Liu X, Zeng W, Wang R, Xiao S (2019) Surfactant-assisted hydrothermal synthesis of V2O5 coated LiNi1/3Co1/3Mn1/3O2 with ideal electrochemical performance. Electrochim Acta 323:134822
Zhou LJ, Yin ZL, Ding ZY, Li XH, Wang ZX, Guo HJ (2017) CeO2 coating to improve the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2. Ionics 24:2533–2542
Dai S, Yan G, Wang L, Luo L, Li Y, Yang Y, Liu H, Liu Y, Yuan M (2019) Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material via CaF2 coating. J Electroanal Chem 847:113197
Kim HB, Park BC, Myung ST, Amine K, Prakash J, Sun YK (2008) Electrochemical and thermal characterization of AlF3-coated Li[Ni0.8Co0.15Al0.05]O2 cathode in lithium-ion cells. J Power Sources 179:347–350
Xiong F, Chen Z, Huang C, Wang T, Zhang W, Yang Z, Chen F (2019) Near-equilibrium control of Li2TiO3 nanoscale layer coated on LiNi0.8Co0.1Mn0.1O2 cathode materials for enhanced electrochemical performance. Inorg Chem 58:15498–15506
Huang Y, Jin FM, Chen FJ, Chen L (2014) Improved cycle stability and high-rate capability of Li3VO4-coated Li[Ni0.5Co0.2Mn0.3]O2 cathode material under different voltages. J Power Sources 256:1–7
Wang JH, Wang Y, Guo YZ, Liu CW, Dan LL (2014) Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met 40:78–83
Liu X, Li H, Li D, Ishida M, Zhou H (2013) PEDOT modified LiNi1/3Co1/3Mn1/3O2 with enhanced electrochemical performance for lithium ion batteries. J Power Sources 243:374–380
Cao G, Jin Z, Zhu J, Li Y, Xu B, Xiong Y, Yang J (2020) A green Al2O3 metal oxide coating method for LiNi0.5Co0.2Mn0.3O2 cathode material to improve the high voltage performance. J Alloys Compd 832:153788
Xiong X, Wang Z, Guo H, Zhang Q, Li X (2013) Enhanced electrochemical properties of lithium-reactive V2O5 coated on the LiNi0.8Co0.1Mn0.1O2 cathode material for lithium ion batteries at 60 ℃. J Mater Chem A 1:1284–1288
Wang D, Li XH, Wang ZX, Guo HJ, Xu Y, Fan YL, Ru JJ (2016) Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim Acta 188:48–56
Li Z, Cao S, Xie X, Wu C, Li H, Chang B, Chen G, Guo X, Zhang X, Wang X (2021) Boosting electrochemical performance of lithium-rich manganese-based cathode materials through a dual modification strategy with defect designing and interface engineering. ACS Appl Mater Interfaces 13:53974–53985
Chen Z, Chao D, Chen M, Shen Z (2020) Hierarchical porous LiNi1/3Co1/3Mn1/3O2 with yolk-shell-like architecture as stable cathode material for lithium-ion batteries. RSC Adv 10:18776–18783
Habibi A, Jalaly M, Rahmanifard R, Ghorbanzadeh M (2020) Microwave-reduced graphene oxide wrapped NCM layered oxide as a cathode material for Li-ion batteries. J Alloy Compd 834:155014
Chen XL, Lu WZ, Chen C, Xue MZ (2018) Improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode with different carbon additives for lithium-ion batteries. Int J Electrochem Sci 13:296–304
Huang B, Wang M, Yang X, Xu G, Gu Y (2019) Enhanced electrochemical performance of the layered nickel-rich oxide cathode by KMnO4 treatment precursor. J Alloy Compd 808:151683
Feng L, Liu Y, Qin W, Yang Z, Liu J (2021) A novel double modification to enhance electrochemical performance of LiNi0.5Co0.2Mn0.3O2 by substituting Ce for Co site. Electrochim Acta 391:138904
Zhang M, Wang C, Zhang J, Li G, Gu L (2021) Preparation and electrochemical characterization of La and Al Co-doped NCM811 cathode materials. ACS Omega 6:16465–16471
Li YC, Zhao WM, Xiang W, Wu ZG, Yang ZG, Xu CL, Xu YD, Wang EH, Wu CJ, Guo XD (2018) Promoting the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via LaAlO3 coating. J Alloy Compd 766:546–555
Zheng H, Chen X, Yang Y, Li L, Li G, Guo Z, Feng C (2017) Self-assembled LiNi1/3Co1/3Mn1/3O2 nanosheet cathode with high electrochemical performance. ACS Appl Mater Interfaces 9:39560–39568
Li Z, Cao S, Wu C, Li H, Chen J, Guo W, Chang B, Shen Y, Bai Y, Wang X (2022) A facile and high-effective oxygen defect engineering for improving electrochemical performance of lithium-rich manganese-based cathode materials. J Power Sources 536:231456
Chen GR, An J, Meng YM, Yuan CZ, Matthews B, Dou F, Shi LY, Zhou Y, Song PG, Wu G, Zhang DS (2019) Cation and anion Co-doping synergy to improve structural stability of Li- and Mn-rich layered cathode materials for lithium-ion batteries. Nano Energy 57:157–165
Zhao LN, Chen GR, Weng YH, Yan TT, Shi LY, An ZX, Zhang DS (2020) Precise Al2O3 Coating on LiNi0.5Co0.2Mn0.3O2 by atomic layer deposition restrains the shuttle effect of transition metals in Li-ion capacitors. Chem Eng J 401:126138
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Zhang, H. & Zhao, W. Improvement of electrochemical performance of LiNi0.5Co0.2Mn0.3O2 by LaF3 coating at high cut-off voltage. Ionics 29, 1335–1345 (2023). https://doi.org/10.1007/s11581-023-04911-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04911-5