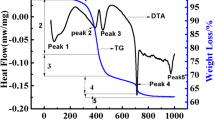
Abstract
Lithium-rich cathode materials have emerged as promising materials for high-energy density lithium-ion cells due to their high specific capacity and high working voltage. In the present work, a comparative study has been made on the thermal stability and electrochemical performance of the lithium-rich cathode material, Li1.5Ni0.25Mn0.75O2.5 (LNMO, synthesized by a co-precipitation method) with commercially available nickel-rich cathode material, LiNi0.8Mn0.1Co0.1O2 (NMC 811). Thermal runaway is a major safety concern hindering the large-scale application of lithium-ion cells in the booming electric vehicle market, and thus the thermal stability of electrode materials has become an important criteria for practical applications. The thermal stability of the cathode materials is investigated by thermogravimetric analysis (TGA). The LNMO cathode material showed better thermal stability in delithiated state than the NMC 811 cathode. A comparative study on the electrochemical performance of both LNMO and NMC 811 cathodes at a working voltage window of 2–4.8 V showed a higher initial discharge capacity (263 mAhg−1) for NMC 811 electrode than LNMO electrode (234 mAhg−1). However, the cycling and rate performance studies indicate excellent performance for LNMO cathode than NMC 811 at the higher working voltage.







Similar content being viewed by others
References
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104(10):4271–4302. https://doi.org/10.1021/cr020731c
Armand M, Tarascon J-M (2008) Building better batteries. Nature 451(7179):652–657. https://doi.org/10.1038/451652a
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4(9):3243–3262. https://doi.org/10.1039/C1EE01598B
Luo Y, Wei H, Tang L, Huang Y, Wang Z, He Z, Yan C, Mao J, Dai K, Zheng J (2022) Nickel-rich and cobalt-free layered oxide cathode materials for lithium ion batteries. Energy Stor Mater 50:274–307. https://doi.org/10.1016/j.ensm.2022.05.019
Schmuch R, Wagner R, Hörpel G, Placke T, Winter M (2018) Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy 3(4):267–278. https://doi.org/10.1038/s41560-018-0107-2
Myung S-T, Maglia F, Park K-J, Yoon CS, Lamp P, Kim S-J, Sun Y-K (2017) Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS Energy Lett 2(1):196–223. https://doi.org/10.1021/acsenergylett.6b00594
Schipper F, Erickson EM, Erk C, Shin J-Y, Chesneau FF, Aurbach D (2016) Review—recent advances and remaining challenges for lithium ion battery cathodes. J Electrochem Soc 164(1):A6220. https://doi.org/10.1149/2.0351701jes
Liu W, Oh P, Liu X, Lee M-J, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed 54(15):4440–4457. https://doi.org/10.1002/anie.201409262
Xu K, von Cresce A (2011) Interfacing electrolytes with electrodes in Li ion batteries. J Mater Chem 21(27):9849–9864. https://doi.org/10.1039/C0JM04309E
Hou PY, Zhang LQ, Gao XP (2014) A High-energy, full concentration-gradient cathode material with excellent cycle and thermal stability for lithium ion batteries. J Mater Chem A 2(40):17130–17138. https://doi.org/10.1039/C4TA03158J
Huang J, Du K, Peng Z, Cao Y, Xue Z, Duan J, Wang F, Liu Y, Hu G (2019) Enhanced high-temperature electrochemical performance of layered nickel-rich cathodes for lithium-ion batteries after LiF surface modification. ChemElectroChem 6(21):5428–5432. https://doi.org/10.1002/celc.201901505
Sun Y, Ren D, Liu G, Mu D, Wang L, Wu B, Liu J, Wu N, He X (2021) Correlation between thermal stabilities of nickel-rich cathode materials and battery thermal runaway. Int J Energy Res 45(15):20867–20877. https://doi.org/10.1002/er.7143
Yu H, Zhou H (2013) High-energy cathode materials (Li2MnO3–LiMO2) for lithium-ion batteries. J Phys Chem Lett 4(8):1268–1280. https://doi.org/10.1021/jz400032v
Zheng J, Myeong S, Cho W, Yan P, Xiao J, Wang C, Cho J, Zhang J-G (2017) Li- and Mn-rich cathode materials: challenges to commercialization. Adv Energy Mater 7(6):1601284. https://doi.org/10.1002/aenm.201601284
Yabuuchi N, Yoshii K, Myung S-T, Nakai I, Komaba S (2011) Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3−LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133(12):4404–4419. https://doi.org/10.1021/ja108588y
Pillai AM, Salini PS, John B, Devassy MT (2022) Aqueous binders for cathodes: a lodestar for greener lithium ion cells. Energy Fuels 36(10):5063–5087. https://doi.org/10.1021/acs.energyfuels.2c00346
Zhou Z, Luo Z, He Z, Zheng J, Li Y, Yan C, Mao J (2021) Suppress voltage decay of lithium-rich materials by coating layers with different crystalline states. J Energy Chem 60:591–598. https://doi.org/10.1016/j.jechem.2021.01.020
Pillai AM, Salini PS, John B, Pillai S, SarojiniAmma S, Mercy T (2023) Synthesis and characterization of Li1.25Ni0.25Mn0.5O2: a high-capacity cathode material with improved thermal stability and rate capability for lithium-ion cells. J Alloys Compd 938:168363. https://doi.org/10.1016/j.jallcom.2022.168363
Yu H, Ishikawa R, So Y-G, Shibata N, Kudo T, Zhou H, Ikuhara Y (2013) Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew Chem Int Ed 52(23):5969–5973. https://doi.org/10.1002/anie.201301236
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J Mater Chem 15(23):2257–2267. https://doi.org/10.1039/B417616M
Thackeray MM, Kang S-H, Johnson CS, Vaughey JT, Hackney SA (2006) Comments on the structural complexity of lithium-rich Li1+xM1−xO2 electrodes (M=Mn, Ni, Co) for lithium batteries. Electrochem Commun 8(9):1531–1538. https://doi.org/10.1016/j.elecom.2006.06.030
Thackeray MM, Kang S-H, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17(30):3112–3125. https://doi.org/10.1039/B702425H
Lu Z, Dahn JR (2002) Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 cells using in situ X-ray diffraction and electrochemical studies. J Electrochem Soc 149(7):A815. https://doi.org/10.1149/1.1480014
Armstrong AR, Holzapfel M, Novák P, Johnson CS, Kang S-H, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J Am Chem Soc 128(26):8694–8698. https://doi.org/10.1021/ja062027
Akhilash M, Salini PS, John B, Mercy TD (2021) A journey through layered cathode materials for lithium ion cells – from lithium cobalt oxide to lithium-rich transition metal oxides. J Alloy Compd 869:159239. https://doi.org/10.1016/j.jallcom.2021.159239
Lanz P, Sommer H, Schulz-Dobrick M, Novák P (2013) Oxygen release from high-energy xLi2MnO3·(1–x)LiMO2 (M=Mn, Ni, Co): electrochemical, differential electrochemical mass spectrometric, in situ pressure, and in situ temperature characterization. Electrochim Acta 93:114–119. https://doi.org/10.1016/j.electacta.2013.01.105
Koga H, Croguennec L, Ménétrier M, Douhil K, Belin S, Bourgeois L, Suard E, Weill F, Delmas C (2013) Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J Electrochem Soc 160(6):A786. https://doi.org/10.1149/2.038306jes
Koga H, Croguennec L, Ménétrier M, Mannessiez P, Weill F, Delmas C (2013) Different oxygen redox participation for bulk and surface: a possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J Power Sources 236:250–258. https://doi.org/10.1016/j.jpowsour.2013.02.075
Pang P, Tan X, Wang Z, Cai Z, Nan J, Xing Z, Li H (2021) Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim Acta 365:137380. https://doi.org/10.1016/j.electacta.2020.137380
Wang Y, Zhang Q, Xue Z-C, Yang L, Wang J, Meng F, Li Q, Pan H, Zhang J-N, Jiang Z, Yang W, Yu X, Gu L, Li H (2020) An in situ formed surface coating layer enabling LiCoO2 with stable 4.6 V high-voltage cycle performances. Adv Energy Mater 10(28):2001413. https://doi.org/10.1002/aenm.202001413
Geder J, Song JH, Kang SH, Yu DYW (2014) Thermal stability of lithium-rich manganese-based cathode. Solid State Ionics 268:242–246. https://doi.org/10.1016/j.ssi.2014.05.020
Akhilash M, Salini PS, Jalaja K, John B, Mercy TD (2021) Synthesis of Li1.5Ni0.25Mn0.75O2.5 cathode material via carbonate co-precipitation method and its electrochemical properties. Inorg Chem Commun 126:108434. https://doi.org/10.1016/j.inoche.2020.108434
Hy S, Felix F, Rick J, Su W-N, Hwang BJ (2014) Direct in situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li[NixLi(1–2x)/3Mn(2–x)/3]O2 (0≤x≤0.5). J Am Chem Soc 136(3):999–1007. https://doi.org/10.1021/ja410137s
Jiang X, Sha Y, Cai R, Shao Z (2015) The solid-state chelation synthesis of LiNi1/3Co1/3Mn1/3O2 as a cathode material for lithium-ion batteries. J Mater Chem A 3(19):10536–10544. https://doi.org/10.1039/C5TA01236H
Zhang X, Jiang WJ, Mauger A, QiluGendron F, Julien CM (2010) Minimization of the cation mixing in Li1+x(NMC)1−xO2 as cathode material. J Power Sources 195(5):1292–1301. https://doi.org/10.1016/j.jpowsour.2009.09.029
Pillai AM, Salini PS, John B, Nair VS, Jalaja K, SarojiniAmma S, Devassy MT (2022) Cobalt-free Li-rich high-capacity cathode material for lithium-ion cells synthesized through sol–gel method and its electrochemical performance. Ionics 28:5005. https://doi.org/10.1007/s11581-022-04725-x
Li N, An R, Su Y, Wu F, Bao L, Chen L, Zheng Y, Shou H, Chen S (2013) The role of yttrium content in improving electrochemical performance of layered lithium-rich cathode materials for Li-ion batteries. J Mater Chem A 1(34):9760–9767. https://doi.org/10.1039/C3TA11665D
Xia Y, Yoshio M, Noguchi H (2006) Improved electrochemical performance of LiFePO4 by increasing its specific surface area. Electrochim Acta 52(1):240–245. https://doi.org/10.1016/j.electacta.2006.05.002
Woo SP, Lee SH, Lee KS, Yoon YS (2014) Effect of increased surface area of LiMn0.475Ni0.475Al0.05O2 cathode material for Li-ion battery. Mater Lett 129:80–83. https://doi.org/10.1016/j.matlet.2014.05.030
Veluchamy A, Doh C-H, Kim D-H, Lee J-H, Shin H-M, Jin B-S, Kim H-S, Moon S-I (2009) Thermal analysis of LixCoO2 cathode material of lithium ion battery. J Power Sources 189(1):855–858. https://doi.org/10.1016/j.jpowsour.2008.07.090
Kim J-S, Johnson CS, Vaughey JT, Thackeray MM, Hackney SA, Yoon W, Grey CP (2004) Electrochemical and structural properties of xLi2M‘O3·(1−x)LiMn0.5Ni0.5O2 electrodes for lithium batteries (M‘ = Ti, Mn, Zr; 0 ≤ x ⩽ 0.3). Chem Mater 16(10):1996–2006. https://doi.org/10.1021/cm0306461
Robertson AD, Bruce PG (2003) Mechanism of electrochemical activity in Li2MnO3. Chem Mater 15(10):1984–1992. https://doi.org/10.1021/cm030047u
Mohanan Pillai A, Salini PS, John B, SarojiniAmma TJ, ThelakkattuDevassy M (2022) Synthesis and electrochemical characterization of a Li-rich Li1.17Ni0.34Mn0.5O2 cathode material for lithium-ion cells. Energy Fuels 36:11186. https://doi.org/10.1021/acs.energyfuels.2c01231
Akhilash M, Salini PS, John B, Mercy TD (2022) The renaissance of high-capacity cathode materials for lithium ion cells. Springer Nature, p 29
Song B, Lai MO, Liu Z, Liu H, Lu L (2013) Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries. J Mater Chem A 1(34):9954–9965. https://doi.org/10.1039/C3TA11580A
Li L, Wang L, Zhang X, Xue Q, Wei L, Wu F, Chen R (2017) 3D reticular Li1.2Ni0.2Mn0.6O2 cathode material for lithium-ion batteries. ACS Appl Mater Interf 9(2):1516–1523. https://doi.org/10.1021/acsami.6b13229
Zhang L, Wu B, Li N, Mu D, Zhang C, Wu F (2013) Rod-like hierarchical nano/micro Li1.2Ni0.2Mn0.6O2 as high performance cathode materials for lithium-ion batteries. J Power Sources 240:644–652. https://doi.org/10.1016/j.jpowsour.2013.05.019
Noh H-J, Youn S, Yoon CS, Sun Y-K (2013) Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J Power Sources 233:121–130. https://doi.org/10.1016/j.jpowsour.2013.01.063
Li X, Peng H, Wang M-S, Zhao X, Huang P-X, Yang W, Xu J, Wang Z-Q, Qu M-Z, Yu Z-L (2016) Enhanced electrochemical performance of Zr-modified layered LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion batteries. ChemElectroChem 3(1):130–137. https://doi.org/10.1002/celc.201500360
Longo RC, Liang C, Kong F, Cho K (2018) Core–shell nanocomposites for improving the structural stability of Li-rich layered oxide cathode materials for Li-ion batteries. ACS Appl Mater Interf 10(22):19226–19234. https://doi.org/10.1021/acsami.8b03898
Acknowledgements
The authors thank Director, Vikram Sarabhai Space Centre, Thiruvananthapuram for granting permission to publish this paper. Analytical Division, Vikram Sarabhai Space Centre is acknowledged for analytical support.
Funding
Akhilash M acknowledges the University of Kerala for granting Junior Research Fellowship (University of Kerala Registration No. AcEVI(2)/718/CHE/18145/2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhilash, M., Salini, P.S., John, B. et al. Thermal stability as well as electrochemical performance of Li-rich and Ni-rich cathode materials—a comparative study. Ionics 29, 983–992 (2023). https://doi.org/10.1007/s11581-022-04873-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04873-0