Abstract
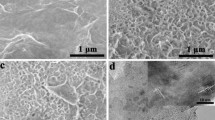
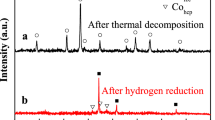
The development of high-performance, cost-effectiveness bifunctional catalysts synthesized by convenient strategy has aroused great attention toward water splitting reaction. In this work, the nanoporous catalyst with a sandwich structure, constructed of amorphous alloy matrix and porous layers, was fabricated by one-step dealloying of Ni25Y60Al15 metallic glass for 15 min in citric acid solution. This catalyst, consisting of the hybrid of NiY and NiO, exhibited a uniform three-dimension porous structure with a pore size of about 5–20 nm. The electrochemical measurements indicated that the nanoporous hybrid possesses excellent catalytic performances toward hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in alkaline medium. The overpotentials at 50 mA/cm2 are about 0.45 V and 0.52 V for HER and OER, respectively, with low Tafel slopes of 43.9 mV/dec and 116.8 mV/dec. The enhanced electro-catalysis mainly contributed to its large electrochemically active surface area and low interfacial charge transfer resistance. As such, this high-performance, low-cost, and bifunctional nanoporous catalyst has broad application prospects in electrocatalytic water splitting.







Similar content being viewed by others
References
Xu Y, Yan Y, He T, Zhan K, Yang JH, Zhao B, Qi K, Xia BY (2019) Supercritical CO2-assisted synthesis of NiFe2O4/vertically-aligned carbon nanotube arrays hybrid as a bifunctional electrocatalyst for efficient overall water splitting. Carbon 145:201–208. https://doi.org/10.1016/j.carbon.2019.01.011
Qian HX, Li KY, Mu XB, Zou JZ, Xie SH, Xiong XB, Zeng XR (2020) Nanoporous NiFeMoP alloy as a bifunctional catalyst for overall water splitting. Int J Hydrog Energy 45:16447–16457. https://doi.org/10.1016/j.ijhydene.2020.04.103
Wang JC, Lin GX, Wang YD, Hong JH, Sun YY (2020) Nano-heterostructures of partially oxidized RuNi alloy as bi-functional electrocatalysts for overall water splitting. Chemsuschem 13:2739–2744. https://doi.org/10.1002/cssc.202000213
Balogun MS, Qiu WT, Huang YC, Yang H, Xu RM, Zhao WX, Li GR, Ji HB, Tong YX (2017) Cost-effective alkaline water electrolysis based on nitrogen- and phosphorus-doped self-supportive electrocatalysts. Adv Mater 29:1702095. https://doi.org/10.1002/adma.201702095
Xia BY, Yan Y, Li N, Wu HB, Lou XW, Wang X (2016) A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat Energy 1:15006. https://doi.org/10.1038/nenergy.2015.6
Yan Y, Xia BY, Zhao B, Wang X (2016) A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J Mater Chem A 4:17587–17603. https://doi.org/10.1039/C6TA08075H
Tu ZY, Zachman MJ, Choudhury S, Wei SY, Ma L, Yang Y, Kourkoutis LF, Archer LA (2017) Nanoporous hybrid electrolytes for high-energy batteries based on reactive metal anodes. Adv Energy Mater 7:1602367. https://doi.org/10.1002/aenm.201602367
Li R, Liu XJ, Wu RY, Wang J, Li ZB, Chan KC, Wang H, Wu Y, Lu ZP (2019) Flexible honeycombed nanoporous/glassy hybrid for efficient electrocatalytic hydrogen generation. Adv Mater 31:1904989. https://doi.org/10.1002/adma.201904989
Dong QC, Sun CC, Dai ZY, Zang XX, Dong XC (2016) Free-standing NiO@C nanobelt as an efficient catalyst for water splitting. ChemCatChem 8:3484–3489. https://doi.org/10.1002/cctc.201601059
Wu HB, Xia BY, Yu L, Yu XY, Lou XW (2015) Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat Commun 6:6512. https://doi.org/10.1038/ncomms7512
Wang Y, Kong B, Zhao D, Wang H, Selomulya C (2017) Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 15:26–55. https://doi.org/10.1016/j.nantod.2017.06.006
Zhao X, Xue ZM, Chen WJ, Wang YQ, Mu TC (2020) Eutectic synthesis of high entropy metal phosphide for electrocatalytic water splitting. Chemsuschem 13:2038–2042. https://doi.org/10.1002/cssc.202000173
Joo J, Kim T, Lee J, Choi S, Lee K (2019) Morphology-controlled metal sulfides and phosphides for electrochemical water splitting. Adv Mater 31:1806682. https://doi.org/10.1002/adma.201806682
Chandrasekaran S, Yao L, Deng LB, Bowen C, Zhang Y, Chen SM, Lin ZQ, Peng F, Zhang PX (2019) Recent advances in metal sulfides: from controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem Soc Rev 48:4178–4280. https://doi.org/10.1039/c8cs00664d
Gao JJ, Tang XW, Du P, Li HL, Yuan QH, Xie GQ, Qiu HJ (2020) Synergistically coupling ultrasmall PtCu nanoalloys with highly porous CoP nanosheets as enhanced electrocatalyst for electrochemical hydrogen evolution. Sustain Energy Fuels 4:2551–2558. https://doi.org/10.1039/C9SE01182J
Zhong X, Wang L, Zhuang ZZ, Chen XL, Zheng J, Zhou YL, Zhuang GL, Li XN, Wang JG (2017) Double nanoporous structure with nanoporous PtFe embedded in graphene nanopores: highly efficient bifunctional electrocatalysts for hydrogen evolution and oxygen reduction. Adv Mater Interfaces 4:1601029. https://doi.org/10.1002/admi.201601029
Ghobrial S, Kirk DW, Thorpe SJ (2019) Amorphous Ni-Nb-Y alloys as hydrogen evolution electrocatalysts. Electrocatalysis-US 10:243–252. https://doi.org/10.1007/s12678-019-00519-4
Shi H, Zhou YT, Yao RQ, Wan WB, Ge X, Zhang W, Wen Z, Lang XY, Zheng WT, Jiang Q (2020) Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat Commun 11:2940. https://doi.org/10.1038/s41467-020-16769-6
Shi H, Zhou YT, Yao RQ, Wan WB, Zhang QH, Gu L, Wen Z, Lang XY, Jiang Q (2020) Intermetallic Cu5Zr Clusters anchored on hierarchical nanoporous copper as efficient catalysts for hydrogen evolution reaction. Research 2020:1–12. https://doi.org/10.34133/2020/2987234
Wang SS, Zhang C, Li HY, Liu L (2017) Enhanced electro-catalytic performance of Pd-based amorphous nanoporous structure synthesized by dealloying Pd32Ni48P20 metallic glass. Intermetallics 87:6–12. https://doi.org/10.1016/j.intermet.2017.04.002
Zheng DH, Zhao F, Li YY, Qin CL, Zhu JS, Hu QF, Wang ZF, Inoue A (2019) Flexible NiO micro-rods/nanoporous Ni/metallic glass electrode with sandwich structure for high performance supercapacitors. Electrochim Acta 297:767–777. https://doi.org/10.1016/j.electacta.2018.12.035
Zhou YY, Liu HP, Zhu SL, Liang YQ, Wu SL, Li ZY, Cui ZD, Chang CT, Yang XJ, Inoue A (2019) Highly efficient and self-standing nanoporous NiO/Al3Ni2 electrocatalyst for hydrogen evolution reaction. ACS Appl Energy Mater 2:7913–7922. https://doi.org/10.1021/acsaem.9b01410
Qin CL, Zheng DH, Hua QF, Zhang XM, Wang ZF, Li YY, Zhu JS, Ou JZ, Yang CH, Wang YC (2020) Flexible integrated metallic glass-based sandwich electrodes for high-performance wearable all-solid-state supercapacitors. Appl Mater Today 19:100539. https://doi.org/10.1016/j.apmt.2019.100539
Wang SS, Li HY, Lin HF, Wu KM (2020) Enhanced electro-catalytic performance of Pd-based hierarchical nanoporous structures fabricated by micropatterning and dealloying of Pd-Ni-P metallic glass. Nanotechnology 31:155301. https://doi.org/10.1088/1361-6528/ab667f
Nesbitt HW, Legrand D, Bancroft GM (2000) Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys Chem Miner 27:357–366. https://doi.org/10.1007/s002690050265
Paolo M, Hernan G, Sanchez C, Federico M, Sarp K, Patricia H-F, Davide D, Hirohito O, Ifan S, Anders N, Ib C (2015) Direct observation of the dealloying process of a platinum–yttrium nanoparticle fuel cell cathode and its oxygenated species during the oxygen reduction reaction. Phys Chem Chem Phys 17:28121–28128. https://doi.org/10.1039/c5cp00283d
Kim J, Shih PC, Tsa KC, Pan YT, Yin X, Sun CJ, Yang H (2017) High-performance pyrochlore-type yttrium ruthenate electrocatalyst for oxygen evolution reaction in acidic media. J Am Chem Soc 139:12076–12083. https://doi.org/10.1021/jacs.7b06808
Jin Y, Xi GG, Li R, Li ZA, Chen XB, Zhang T (2021) Nanoporous metallic-glass electrocatalysts for highly efficient oxygen evolution reaction. J Alloy Compd 852:156876. https://doi.org/10.1016/j.jallcom.2020.156876
Ren XR, Zhai YY, Zhou Q, Yan JQ, Liu SZ (2020) Fabrication of nanoporous Ni and NiO via a dealloying strategy for water oxidation catalysis. J Energy Chem 50:125–134. https://doi.org/10.1016/j.jechem.2020.03.020
Li YG, Wang HL, Xie LM, Liang YY, Hong GS, Dai HJ (2011) MoS2 Nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J Am Chem Soc 133:7296–7299. https://doi.org/10.1021/ja201269b
Merki D, Vrubel H, Rovelli L, Fierro S, Hu XL (2012) Fe Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem Sci 3:2515–2525. https://doi.org/10.1039/C2SC20539D
Xu WC, Zhu SL, Liang YQ, Cui ZD, Yang XJ, Inoue A (2018) Nanoporous metal phosphide catalyst for bifunctional water splitting. J Mater Chem A 6:5574–5579. https://doi.org/10.1039/x0xx00000x
Du YM, Han Y, Huai XD, Liu YD, Wu CY, Yang Y, Wang L (2018) N-doped carbon coated FeNiP nanoparticles based hollow microboxes for overall water splitting in alkaline medium. Int J Hydrogen Energ 43:22226–22234. https://doi.org/10.1016/j.ijhydene.2018.10.091
Li N, Bediako DK, Hadt RG, Hayes D, Kempa TJ, Cube FV, Bell DC, Chen LX, Nocera DG (2017) Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. P Natl Acad Sci USA 114:1486–1491. https://doi.org/10.1073/pnas.1620787114
Trotochaud L, Ranney JK, Williams KN, Boettcher SW (2012) Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J Am Chem Soc 134:17253–17261. https://doi.org/10.1021/ja307507a
Wang J, Li Y, Li ZB, Li LXJ, R, Du Q, Wang XZ, Wang H, Wu Y, Jiang SH, Lu ZP, (2021) Self-supporting nanoporous Ni/metallic glass composites with hierarchically porous structure for efficient hydrogen evolution reaction. J Mater Sci Technol 73:145–150. https://doi.org/10.1016/j.jmst.2020.09.016
Suen NT, Hung SF, Quan Q, Zhang N, Xu YJ, Chen HM (2017) Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev 46:337–365. https://doi.org/10.1039/c6cs00328a
Guan BY, Yu L, Lou XW (2017) General synthesis of multishell mixed-metal oxyphosphide particles with enhanced electrocatalytic activity in the oxygen evolution reaction. Angew Chem Int Edit 56:2386–2389. https://doi.org/10.1002/anie.201611804
Ledendecker M, Papp C, Steinruck HP, Antonietti M, Shalom M (2015) The synthesis of nanostructured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew Chem Int Edit 127:12538–12542. https://doi.org/10.1002/anie.201502438
Zhang YQ, Ouyang B, Xu J, Jia GC, Chen S, Rawat RS, Fan HJ (2016) Rapid synthesis of cobalt nitride nanowires: highly efficient and low-cost catalysts for oxygen evolution. Angew Chem Int Edit 128:8812–8816. https://doi.org/10.1002/anie.201604372
Al-Hoshan MS, Singh JP, Al-Mayouf AM, Al-Suhybani AA, Shaddad MN (2012) Synthesis, physicochemical and electrochemical properties of nickel ferrite spinels obtained by hydrothermal method for the oxygen evolution reaction (OER). Int J Electrochem Sci 7:4959–4973. https://doi.org/10.1002/fuce.201100111
Mondal A, Paul A, Srivastava DN, Panda AB (2018) NiO hollow microspheres as efficient bifunctional electrocatalysts for Overall Water-Splitting. Int J Hydrogen Energ 43:21665–21674. https://doi.org/10.1016/j.ijhydene.2018.06.139
Ravi DC, Esther SMS, Jothivenkatachalam K, Joseph NA, Kavinkumar V, Arivukarasan VR, D, (2021) Direct-grown nebulizer-sprayed nickel-copper mixed metal oxide nanocomposite films as bifunctional electrocatalyst for water splitting. Ionics. https://doi.org/10.1007/s11581-021-04285-6
Yang F, Kang N, Yan JY, Wang XL, He J, Huo SY, Song LZ (2018) Hydrogen evolution reaction property of molybdenum disulfide/nickel phosphide hybrids in alkaline solution. Metals-Basel 8:359. https://doi.org/10.3390/met8050359
Glasscott MW, Pendergast AD, Goines S, Bishop AR, Hoang AT, Renault C, Dick JE (2019) Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat Commun 10:2650. https://doi.org/10.1038/s41467-019-10303-z
Oscar DM, Isis LY, Koper MTM, Federico CV (2015) Guidelines for the rational design of Ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal 9:5380–5387. https://doi.org/10.1021/acscatal.5b01638
Voiry D, Chhowalla M, Gogotsi Y, Kotov NA, Li Y, Penner RM, Schaak RE, Weiss PS (2018) Best practices for reporting electrocatalytic performance of nanomaterials. ACS Nano 12:9635–9638. https://doi.org/10.1021/acsnano.8b07700
Kichigin VI, Shein AB (2018) An electrochemical study of the hydrogen evolution reaction at YNi2Ge2 and LaNi2Ge2 electrodes in alkaline solution. J Electroanal Chem 830–831:2–79. https://doi.org/10.1016/j.jelechem.2018.10.029
Rosalbino F, Borzone G, Angelini E, Raggio R (2003) Hydrogen evolution reaction on Ni-RE (RE = rare earth) crystalline alloys. Electrochim Acta 48:3939–3944. https://doi.org/10.1016/S0013-4686(03)00532-2
Ye SH, Shi ZX, Feng JX, Tong YX, Li GR (2018) Activating CoOOH porous nanosheet arrays by partial iron substitution for efficient oxygen evolution reaction. Angew Chem Int Edit 57:2672–2676. https://doi.org/10.1002/anie.201712549
Acknowledgements
The authors greatly acknowledge the financial support from the State Key Lab of Advanced Metals and Materials (Grant No. 2019-Z04), National Natural Science Foundation of China (Grant Nos. U20A20279, 11804260, 11704292), and Hubei Province Key Laboratory of Systems Science in Metallurgical Process (Grant No. Y201903). We would like to thank Mr. Zhang at the Analytical & Testing Center of Wuhan University of Science and Technology for the help on XPS analysis.
This is the first submission of this manuscript and no parts of this manuscript are being considered for publication elsewhere. All authors have approved this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, F., Wang, S., Gu, L. et al. One-step dealloying of Ni-Y-Al metallic glass for fabrication of nanoporous hybrid toward efficient water splitting reaction. Ionics 28, 1367–1376 (2022). https://doi.org/10.1007/s11581-021-04434-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04434-x