Abstract
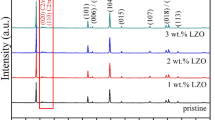
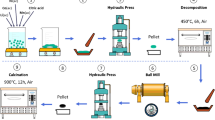
In this paper, the sol-gel method was used first to obtain the layered cathode material Li1.2Mn0.54Ni0.13Co0.13O2, and then the nanoparticles of TiO2 were coated samples in via a wet chemical process. Finally, a systematic study of the different amounts of TiO2 materials coated was carried out. XRD and SEM analysis showed that these materials after TiO2 coating have a good layered structure and regular morphology, respectively. Experiments such as XPS and TEM showed that the TiO2 nanoparticles were evenly distributed on the surface of the particles, and no substantial changes were made to each transition metal elements. Through electrochemical testing, when the TiO2 coating amount is equal to 1.0%, the first discharge-specific capacity reaches 276.5 mAh/g at 0.1 C, and the Coulomb efficiency is also as high as 80.8%. Compared with the uncoated sample, when the coating amount is 1.0%, the TiO2 coating suppresses the lack of surface oxygen and makes the structure more stable. The electrochemical performance of the material has been significantly improved.












Similar content being viewed by others
References
Liu H, Chen C, Du C, He X, Yin G, Song B, Zuo P, Cheng X, Ma Y, Gao Y (2015) Lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 oxide coated by Li3PO4 and carbon nanocomposite layers as high performance cathode materials for lithium ion batteries. J Mater Chem A 3:2634–2641
Yu S-Z, Luo S-H, Zhan Y, Huang H-B, Wang Q, Zhang Y-H, Liu Y-G, Hao A-I (2020) Metal-organic framework-derived cobalt nanoparticle space confined in nitrogen-doped carbon polyhedra networks as high-performance bifunctional electrocatalyst for rechargeable Li–O2 batteries. J Power Sources 453:227899–227911
Yin H, Ji S, Gu M, Zhang L, Liu J (2015) Scalable synthesis of Li1.2Mn0.54Ni0.13Co0.13O2/LiNi0.5Mn1.5O4 sphere composites as stable and high capacity cathodes for Li-ion batteries. RSC Adv 5:84673–84679
Su WX, Feng WJ, Wang S, Chen L, Li M, Song C (2019) Electrocatalysis of polysulfide conversion via sulfur–cobalt CoS2 on a carbon nanotube surface as a cathode for high-performance lithium–sulfur batteries. J Solid State Electrochem 23:2097–2105
Luo S-H, Sun Y, Bao S, Li J-Z, Zhang J, Yi T-F (2019) Synthesis of Er-doped LiMnPO4/C by a sol-assisted hydrothermal process with superior rate capability. J Electroanal Chem 832:196–203
Wu F, Wang Z, Su Y, Yan N, Bao L, Chen S (2014) Li[Li0.2Mn0.54Ni0.13Co0.13]O2 –MoO3 composite cathodes with low irreversible capacity loss for lithium ion batteries. J Power Sources 247:20–25
Li B, Yan H, Ma J, Yu P, Xia D, Huang W, Chu W, Wu Z (2014) Manipulating the electronic structure of Li-rich manganese-based oxide using polyanions: towards better electrochemical performance. Adv Funct Mater 24:5112–5118
Liu Y, Wang H, Yang K, Yang Y, Ma J, Pan K, Wang G, Ren F, Pang H (2019) Enhanced electrochemical performance of Sb2O3 as an anode for lithium-ion batteries by a stable cross-linked binder. Appl Sci Basel 9:2677–2686
Dai D, Li B, Tang H, Chang K, Jiang K, Chang Z, Yuan X (2016) Simultaneously improved capacity and initial coulombic efficiency of Li-rich cathode Li[Li0.2Mn0.54Co0.13Ni0.13]O2 by enlarging crystal cell from a nanoplate precursor. J Power Sources 307:665–672
Kong J-Z, Zhai H-F, Qian X, Wang M, Wang Q-Z, Li A-D, Li H, Zhou F (2017) Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material coated with ultrathin ZnO. J Alloys Compd 694:848–856
Bai Y, Li Y, Wu C, Lu J, Li H, Liu Z, Zhong Y, Chen S, Zhang C, Amine K, Wu F (2015) Lithium-rich nanoscale Li1.2Mn0.54Ni0.13Co0.13O2 cathode material prepared by co-precipitation combined freeze drying (CP-FD) for lithium-ion batteries. Energy Technol 3:843–850
Li M, Feng W, Wenxiao S, Wang X (2019) CoNi-embedded nitrogen-enriched porous carbon framework for long-life lithium–sulfur batteries. J Solid State Electrochem 23:2317–2324
Wang Z, Luo S, Ren J, Wang D, Qi X (2016) Enhanced electrochemical performance of Li-rich cathode Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification with lithium ion conductor Li3PO4. Appl Surf Sci 370:437–444
Zhang J, Wang J, Yang J, NuLi Y (2014) Artificial interface deriving from sacrificial tris(trimethylsilyl)phosphate additive for lithium rich cathode materials. Electrochim Acta 117:99–104
Zhang L, Jiang J, Zhang C, Wu B, Wu F (2016) High-rate layered lithium-rich cathode nanomaterials for lithium-ion batteries synthesized with the assist of carbon spheres templates. J Power Sources 331:247–257
Li J, Jia T, Liu K, Zhao J, Chen J, Cao C (2016) Facile design and synthesis of Li-rich nanoplates cathodes with habit-tuned crystal for lithium ion batteries. J Power Sources 333:37–42
Tang T, Zhang H-L (2016) Synthesis and electrochemical performance of lithium-rich cathode material Li[Li0.2Ni0.15Mn0.55Co0.1-xAlx]O2. Electrochim Acta 191:263–269
Thackeray MM, Johnson CS, Vaughey JT, Li N (2005) Current address: eVionyx Inc., Ha, S.A. Hackney, Advances in manganese-oxide “composite” electrodes for lithium-ion batteries. J Mater Chem 15:2257
Thackeray MM, Kang S-H, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2(M=Mn,Ni,Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112
Liu X, Liu J, Huang T, Yu A (2013) CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries. Electrochim Acta 109:52–58
Liu Y, Yang Z, Li J, Niu B, Yang K, Kang F (2018) A novel surface-heterostructured Li1.2Mn0.54Ni0.13Co0.13O2 @Ce0.8Sn0.2O2−σ cathode material for Li-ion batteries with improved initial irreversible capacity loss. J Mater Chem A 6:13883–13893
Wang M, Luo M, Chen Y, Chen L, Yan S, Ren Y, Chu M (2017) A new approach to improve the electrochemical performance of Li-rich cathode material by precursor pretreatment. J Alloys Compd 696:891–899
Chen JJ, Li ZD, Xiang HF, Wu WW, Cheng S, Zhang LJ, Wang QS, Wu YC (2015) Enhanced electrochemical performance and thermal stability of a CePO4-coated Li1.2Ni0.13Co0.13Mn0.54O2 cathode material for lithium-ion batteries. RSC Adv 5:3031–3038
Yi T-F, Mei J, Peng P-P, Luo S-H (2019) Facile synthesis of polypyrrole-modified Li5Cr7Ti6O25 with improved rate performance as negative electrode material for Li-ion batteries. Compos Part B 167:566–572
Chen J, Wang X, Tang F, Ye X, Yang L, Zhang Y (2020) Substrates for surface-enhanced Raman spectroscopy based on TiN plasmonic antennas and waveguide platforms. Results Phys 16:102867
Wang C-C, Lin J-W, Yu Y-H, Lai K-H, Chiu K-F, Kei C-C (2018) Electrochemical and structural investigation on ultrathin ALD ZnO and TiO2 coated lithium-rich layered oxide cathodes. ACS Sustain Chem Eng 6:16941–16950
Ran X-X, Tao J-M, Chen Z-Y, Yan Z-R, Yang Y-M, Li J-X, Lin Y-B, Huang Z (2020) Surface heterostructure induced by TiO2 modification in Li-rich cathode materials for enhanced electrochemical performances. Electrochim Acta 353:135959
Gan Y, Wang Y, Han J, Zhang L, Sun W, Xia Y, Huang H, Zhang J, Liang C, Zhang W (2017) Synthesis and electrochemical performance of nano TiO2(B)-coated Li[Li0.2Mn0.54Co0.13Ni0.13]O2 cathode materials for lithium-ion batteries. New J Chem 41:12962–12968
Yuan X, Xu Q, Liu X, Liu H, Min Y, Xia Y (2016) Layered cathode material with improved cycle performance and capacity by surface anchoring of TiO2 nanoparticles for Li-ion batteries. Electrochim Acta 213:648–654
Xiang Y, Sun Z, Li J, Wu X, Liu Z, Xiong L, He Z, Long B, Yang C, Yin Z (2017) Improved electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode material for lithium ion batteries synthesized by the polyvinyl alcohol assisted sol-gel method. Ceram Int 43:2320–2324
Ma D, Li Y, Zhang P, Cooper AJ, Abdelkader AM, Ren X, Deng L (2016) Mesoporous Li1.2Mn0.54Ni0.13Co0.13O2 nanotubes for high-performance cathodes in Li-ion batteries. J Power Sources 311:35–41
Jiao LF, Zhang M, Yuan HT, Zhao M, Guo J, Wang W, Zhou XD, Wang YM (2007) Effect of Cr doping on the structural, electrochemical properties of Li[Li0.2Ni0.2−x/2Mn0.6−x/2Crx]O2(x=0, 0.02, 0.04, 0.06, 0.08) as cathode materials for lithium secondary batteries. J Power Sources 167:178–184
Zheng JM, Li J, Zhang ZR, Guo XJ, Yang Y (2008) The effects of TiO2 coating on the electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium-ion battery. Solid State Ionics 179:1794–1799
Lu C, Yang S, Wu H, Zhang Y, Yang X, Liang T (2016) Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium doping. Electrochim Acta 209:448–455
Zhang L, Jin K, Wang L, Zhang Y, Li X, Song Y (2015) High capacity Li1.2Mn0.54Ni0.13Co0.13O2 cathode materials synthesized using mesocrystal precursors for lithium-ion batteries. J Alloys Compd 638:298–304
Song CK, Feng WJ, Su WX, Chen LJ, Li MM (2019) Influence of the pH of li-rich Li1.2Mn0.54Ni0.13Co0.13O2 on the electrochemical performance by sol–gel method. Integr Ferroelectr 200:117–127
Wang C, Zhou F, Chen K, Kong J, Jiang Y, Yan G, Li J, Yu C, Tang W-P (2015) Electrochemical properties of α-MoO3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. Electrochim Acta 176:1171–1181
Xu H, Deng S, Chen G (2014) Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Mg doping for lithium ion battery cathode material. J Mater Chem A 2:15015–15021
Jin X, Xu Q, Liu H, Yuan X, Xia Y (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim Acta 136:19–26
Liu J, Hou M, Yi J, Guo S, Wang C, Xia Y (2014) Improving the electrochemical performance of layered lithium-rich transition-metal oxides by controlling the structural defects. Energy Environ Sci 7:705–714
Song C, Feng W, Shi Z, Wang X (2019) Effect of drying temperature on properties of lithium-rich manganese-based materials in sol-gel method. Ionics. 25:4607–4614
Deng Y-P, Fu F, Wu Z-G, Yin Z-W, Zhang T, Li J-T, Huang L, Sun S-G (2016) Layered/spinel heterostructured Li-rich materials synthesized by a one-step solvothermal strategy with enhanced electrochemical performance for Li-ion batteries. J Mater Chem A 4:257–263
Kong J-Z, Wang C-L, Qian X, Tai G-A, Li A-D, Wu D, Li H, Zhou F, Yu C, Sun Y, Jia D, Tang W-P (2015) Enhanced electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by surface modification with graphene-like lithium-active MoS2. Electrochim Acta 174:542–550
Xue Q, Li J, Xu G, Zhou H, Wang X, Kang F (2014) In situ polyaniline modified cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with high rate capacity for lithium ion batteries. J Mater Chem A 2:18613–18623
Yan W, Xie Y, Jiang J, Sun D, Ma X, Lan Z, Jin Y (2018) Enhanced rate performance of Al-doped Li-rich layered cathode material via nucleation and post-solvothermal method. ACS Sustain Chem Eng 6:4625–4632
Zhao Y, Xia M, Hu X, Zhao Z, Wang Y, Lv Z (2015) Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.8O2 Li-rich cathode materials. Electrochim Acta 174:1167–1174
Yi T-F, Qiu L-Y, M J, Qi S-Y, Cui P, Luo S-H, Zhu Y-R, Xie Y, He Y-B (2020) Porous spherical NiO@NiMoO4@PPy nanoarchitectures as advanced electrochemical pseudocapacitor materials. Sci Bull 65:546–556
Xu G, Li J, Li X, Zhou H, Ding X, Wang X, Kang F (2015) Understanding the electrochemical superiority of 0.6Li[Li1/3Mn2/3]O2-0.4Li[Ni1/3Co1/3Mn1/3]O2 nanofibers as cathode material for lithium ion batteries. Electrochim Acta 173:672–679
Yan J, Liu X, Li B (2014) Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv 4:63268–63284
Feng X, Yang Z, Tang D, Kong Q, Gu L, Wang Z, Chen L (2015) Performance improvement of Li-rich layer-structured Li1.2Mn0.54Ni0.13Co0.13O2 by integration with spinel LiNi0.5Mn1.5O4. Phys Chem Chem Phys 17:1257–1264
Zhou Y, Bai P, Tang H, Zhu J, Tang Z (2016) Chemical deposition synthesis of desirable high-rate capability Al2O3-coated Li1.2Mn0.54Ni0.13Co0.13O2 as a lithium ion battery cathode material. J Electroanal Chem 782:256–263
Song CK, Feng WJ, Su WX, Chen LJ, Li MM (2019) Effect of drying time on electrochemical properties of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. Int J Electrochem Sci 14:2372–2382
Mohadese R-D, Mehran J, Hamid O (2019) Enhanced performance of layered Li1.2Mn0.54Ni0.13Co0.13O2 cathode material in Li-ion batteries using nanoscale surface coating with fluorine-doped anatase TiO2. Solid State Ionics 331:74–88
Nayak PK, Grinblat J, Levi M, Levi E, Kim S, Choi JW, Aurbach D (2016) Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv Energy Mater 6:1502398
Yi T-F, Li Y-M, Li X-Y, Pan J-J, Zhang Q, Zhu Y-R (2017) Enhanced electrochemical property of FePO4-coated LiNi0.5Mn1.5O4 as cathode materials for Li-ion battery. Sci Bull 62:1007–1010
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No.21965019), HongLiu First-class Disciplines Development Program of Lanzhou University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, C., Feng, W., Shi, Z. et al. Coating TiO2 on lithium-rich Li1.2Mn0.54Ni0.13Co0.13O2 material to improve its electrochemical performance. Ionics 27, 457–468 (2021). https://doi.org/10.1007/s11581-020-03854-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03854-5