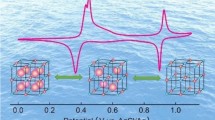
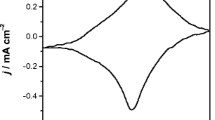
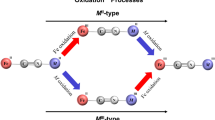
Abstract
Prussian blue and its analogues are well known nowadays as promising substances for energy storage, capable of electrochemical insertion of Li+, Na+, Ca2+, and other ions. A huge amount of experimental data obtained recently is in evident contradiction with the postulates declared in the early investigations on Prussian blue electrochemistry. Nevertheless, some of these old postulates are widely in use up to now. On the basis of the data on the chemistry and the composition of the Prussian blue not previously involved in the discussion, this article examines the possible participation of hydrated ions in the redox process, the composition of the electrodeposited films of this compound, and some other important problems. Analytical data on Prussian blue, obtained in the nineteenth century and underestimated by twentieth century chemists, suggests that it is a non-stoichiometric compound. Equations describing electrochemical processes of this substance have been proposed.


Similar content being viewed by others
References
Keggin JF, Miles FD (1936) Structures and formulæ of the Prussian blues and related compounds. Nature 137:577–578. https://doi.org/10.1038/137577a0
You Y, Wu XL, Yin YX, Guo YG (2014) High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ Sci 7:1643–1647. https://doi.org/10.1039/C3EE44004D
Pasta M, Wessells CD, Liu N, Nelson J, McDowell MT, Huggins RA, Toney MF, Cui Y (2014) Full open-framework batteries for stationary energy storage. Nat Commun 5:3007. https://doi.org/10.1038/ncomms4007
Wang B, Han Y, Wang X, Bahlawane N, Pan H, Yan M, Yan M, Jiang Y (2018) Prussian blue analogs for rechargeable batteries. iScience 3:110–133. https://doi.org/10.1016/j.isci.2018.04.008
Xu Y, Zheng S, Tang H, Guo X, Xue H, Pang H (2017) Prussian blue and its derivatives as electrode materials for electrochemical energy storage. Energy Storage Mater 9:11–30. https://doi.org/10.1016/j.ensm.2017.06.002
Rudola A, Du K, Balaya P (2017) Monoclinic sodium iron hexacyanoferrate cathode and non-flammable glyme-based electrolyte for inexpensive sodium-ion batteries. J Electrochem Soc 164:A1098–A1109. https://doi.org/10.1149/2.0701706jes
Wang L, Song J, Qiao R, Wray LA, Hossain MA, Chuang Y-D, Yang W, Lu Y, Evans D, Lee J-J, Vail S, Zhao X, Nishijima M, Kakimoto S, Goodenough JB (2015) Rhombohedral Prussian white as cathode for rechargeable sodium-ion batteries. J Am Chem Soc 137:2548–2554. https://doi.org/10.1021/ja510347s
Zhou L, Yang Z, Li C, Chen B, Wang Y, Fu L, Zhu Y, Liu X, Wu Y (2016) Prussian blue as positive electrode material for aqueous sodium-ion capacitor with excellent performance. RSC Adv 6:109340–109345. https://doi.org/10.1039/C6RA21500A
Kim H, Kim H, Ding Z, Lee MH, Lim K, Yoon G, Kang K (2016) Recent progress in electrode materials for sodium-ion batteries. Adv Energy Mater 6:1600943. https://doi.org/10.1002/aenm.201600943
Paolella A, Faure C, Timoshevskii V, Marras S, Bertoni G, Guerfi A, Vijh A, Armand M, Zaghib K (2017) A review on hexacyanoferrate-based materials for energy storage and smart windows: challenges and perspectives. J Mater Chem A 5:18919–18932. https://doi.org/10.1039/C7TA05121B
Mullaliu A, Giorgetti M (2019) Metal hexacyanoferrates: ion insertion (or exchange) capabilities. In: Inamuddin AM, Asiri A (eds) Applications of ion exchange materials in the environment. Springer, Cham, pp 109–133. https://doi.org/10.1007/978-3-030-10430-6_6
Neff VD (1978) Electrochemical oxidation and reduction of thin films of Prussian blue. J Electrochem Soc 125:886–887. https://doi.org/10.1149/1.2131575
Itaya K, Uchida I, Neff VD (1986) Electrochemistry of polynuclear transition metal cyanides: Prussian blue and its analogues. Acc Chem Res 19:162–168. https://doi.org/10.1021/ar00126a001
Mortimer RJ, Rosseinsky DR (1984) Iron hexacyanoferrate films: spectroelectrochemical distinction and electrodeposition sequence of ‘soluble’ (K+-containing) and ‘insoluble’ (K+-free) Prussian blue, and composition changes in polyelectrochromic switching. J Chem Soc Dalton Trans:2059–2062. https://doi.org/10.1039/DT9840002059
Porrett R (1814) XXVI. On the nature of the salts termed triple prussiates, and on acids formed by the union of certain bodies with the elements of the prussic acid. Phil Trans R Soc Lond 104:527–556. https://doi.org/10.1098/rstl.1814.0027
Yang R, Qian Z, Deng J (1998) Electrochemical deposition of Prussian blue from a single ferricyanide solution. J Electrochem Soc 145:2231–2236. https://doi.org/10.1149/1.1838625
Liebig J, Geiger PL (1843) Handbuch der Chemie : Mit Rücksicht auf Pharmacie; Als neue Bearbeitung des ersten Bandes von Geiger's Handbuch der Pharmacie, Heidelberg 2, S. 642 The book is available at https://reader.digitale-sammlungen.de/de/fs1/object/display/bsb10702759_00050.html (accessed 16 June 2019)
Rigamonti R (1937) Struttura dei cupriferricianuri. – Nota I. Ferrocianuro dì rame e ferrocianuri di rame e potassio. Gazz chim It 67:137–146
Rigamonti R (1938) Struttura e costituzione chimica di alcuni ferrocianuri. Gazz chim It 68:803–809
Tananaev IV, Seifer GB, Kharitonov YY, Kuznetsov VG, Korol’kov AP (1971) Khimiya ferrocyanidov (Chemistry of ferrocyanides, in Russian). Nauka, Moscow
Emeléus HJ, Anderson JS (1939) Modern aspects of inorganic chemistry. D. Van Nostrand Company, New York, p 137
Jassal V, Shanker U, Shankar S (2015) Synthesis, characterization and applications of nano-structured metal hexacyanoferrates: a review. J Environ Anal Chem 2:1000128. https://doi.org/10.4172/2380-2391.1000128
Huggins RA (2016) Energy storage for medium- to large-scale applications. In: Energy storage. Springer, Cham, pp 427–471. https://doi.org/10.1007/978-3-319-21239-5_22
Kuwabara K, Nunome J, Sugiyama K (1991) Rechargeability of solid-state copper cells utilizing cathodes of Prussian blue and Berlin green. Solid State lonics 48:303–308. https://doi.org/10.1016/0167-2738(91)90047-F
Buser HJ, Schwarzenbach D, Petter W, Ludi A (1977) The crystal structure of Prussian blue: Fe4[Fe(CN)6]3∙xH2O. Inorg Chem 16:2704–2710. https://doi.org/10.1021/ic50177a008
Kulesza PJ, Malik MA, Berrettoni M, Giorgetti M, Zamponi S, Schmidt R, Marassi R (1998) Electrochemical charging, countercation accommodation, and spectrochemical identity of microcrystalline solid cobalt hexacyanoferrate. J Phys Chem B 102:1870–1876. https://doi.org/10.1021/jp9726495
Herren F, Fischer P, Ludi A, Haelg W (1980) Neutron diffraction study of Prussian blue, Fe4[Fe(CN)6]3∙xH2O. Location of water molecules and long-range magnetic order. Inorg Chem 19:956–959. https://doi.org/10.1021/ic50206a032
Vol’khin VV, Shul’ga EA, Zil’berman MV (1971) Ion-exchange properties of mixed ferrocyanides of some transition metals (in Russian). Izv Akad Nauk SSSR Neorg Mater 7:77–81
Lundgren CA, Murray RW (1988) Observations on the composition of Prussian blue films and their electrochemistry. Inorg Chem 27:933–939. https://doi.org/10.1021/ic00278a036
Kulesza PJ, Zamponi S, Berrettoni M, Marassi R, Malik MA (1995) Preparation, spectroscopic characterization and electrochemical charging of the sodium-containing analogue of Prussian blue. Electrochim Acta 40:681–688. https://doi.org/10.1016/0013-4686(94)00348-5
Kulesza PJ, Zamponi S, Malik MA, Miecznikowski K, Berrettoni M, Marassi R (1997) Spectroelectrochemical identity of Prussian blue films in various electrolytes: comparison of time-derivative voltabsorptometric responses with conventional cyclic voltammetry. J Solid State Electrochem 1:88–93. https://doi.org/10.1007/s100080050027
García-Jareño JJ, Sanmatías A, Vicente F, Gabrielli C, Keddam M, Perrot H (2000) Study of Prussian blue (PB) films by ac-electrogravimetry: influence of PB morphology on ions movement. Electrochim Acta 45:3765–3776. https://doi.org/10.1016/S0013-4686(00)00470-9
Feldman BJ, Melroy OR (1987) Ion flux during electrochemical charging of Prussian blue films. J Electroanal Chem 234:213–227. https://doi.org/10.1016/0022-0728(87)80173-0
Dostal A, Meyer B, Scholz F, Schröder U, Bond AM, Marken F, Shaw SJ (1995) Electrochemical study of microcrystalline solid Prussian blue particles mechanically attached to graphite and gold electrodes: electrochemically induced lattice reconstruction. J Phys Chem 99:2096–2103. https://doi.org/10.1021/j100007a045
Zadronecki M, Wrona PK, Galus Z (1999) Study of growth and the electrochemical behavior of Prussian blue films using electrochemical quartz crystal microbalance. J Electrochem Soc 146:620–627. https://doi.org/10.1149/1.1391653
Oh I, Lee H, Yang H, Kwak J (2001) Ion and water transports in Prussian blue films investigated with electrochemical quartz crystal microbalance. Electrochem Commun 3:274–280. https://doi.org/10.1016/S1388-2481(01)00144-8
Ogura K, Nakayama M, Nakaoka K (1999) Electrochemical quartz crystal microbalance and in situ infrared spectroscopic studies on the redox reaction of Prussian blue. J Electroanal Chem 474:101–106. https://doi.org/10.1016/S0022-0728(99)00306-X
Housecroft CE, Sharpe AG (2012) Inorganic chemistry, 4th. Pearson Education Limited
Marcus Y (2012) Are ionic stokes radii of any use? J Solut Chem 41:2082–2090. https://doi.org/10.1007/s10953-012-9922-4
Nightingale ER (1959) Phenomenological theory of ion solvation. Effective radii of hydrated ions. J Phys Chem 63:1381–1387. https://doi.org/10.1021/j150579a011
Lee C, Jeong S-K (2018) Modulating the hydration number of calcium ions by varying the electrolyte concentration: electrochemical performance in a Prussian blue electrode/aqueous electrolyte system for calcium-ion batteries. Electrochim Acta 265:430–436. https://doi.org/10.1016/j.electacta.2018.01.172
Kunz W, Lo Nostro P, Ninham BW (2004) The present state of affairs with Hofmeister effects. Curr Opin Colloid Interface Sci 9:1–18. https://doi.org/10.1016/j.cocis.2004.05.004
Jenny H (1932) Studies on the mechanism of ionic exchange in colloidal aluminum silicates. J Phys Chem 36:2217–2258. https://doi.org/10.1021/j150338a011
Gregor HP, Gutoff F, Bregman JI (1951) Studies on ion-exchange resins. II. Volumes of various cation-exchange resin particles. J Colloid Sci 6:245–270. https://doi.org/10.1016/0095-8522(51)90043-8
Pauley JL (1954) Prediction of cation-exchange equilibria. J Am Chem Soc 76:1422–1425. https://doi.org/10.1021/ja01634a085
Itaya K, Ataka T, Toshima S (1982) Spectroelectrochemistry and electrochemical preparation method of Prussian blue modified electrodes. J Am Chem Soc 104:4767–4772. https://doi.org/10.1021/ja00382a006
Bocarsly AB, Sinha S (1982) Chemically-derivatized nickel surfaces: synthesis of a new class of stable electrode interfaces. J Electroanal Chem 137:157–162. https://doi.org/10.1016/0022-0728(82)85075-4
Bocarsly AB, Sinha S (1982) Effects of surface structure on electrode charge transfer properties: induction of ion selectivity at the chemically derivatized interface. J Electroanal Chem 140:167–172. https://doi.org/10.1016/0368-1874(82)85310-0
Sinha S, Humphrey BD, Bocarsly AB (1984) Reaction of nickel electrode surfaces with anionic metal-cyanide complexes: formation of precipitated surfaces. Inorg Chem 23:203–212. https://doi.org/10.1021/ic00170a018
Kelly MT, Arbuckle-Keil GA, Johnson LA, Su EY, Amos LJ, Chun JKM, Bocarsly AB (2001) Nickel ferrocyanide modified electrodes as active cation-exchange matrices: real time XRD evaluation of overlayer structure and electrochemical behavior. J Electroanal Chem 500:311–321. https://doi.org/10.1016/S0022-0728(00)00487-3
Schneemeyer LF, Spengler SE, Murphy DW (1985) Ion selectivity in nickel hexacyanoferrate films on electrode surfaces. Inorg Chem 24:3044–3046. https://doi.org/10.1021/ic00213a034
Bácskai J, Martinusz K, Czirók E, Inzelt G, Kulesza PJ, Malik MA (1995) Polynuclear nickel hexacyanoferrates: monitoring of film growth and hydrated counter-cation flux/storage during redox reactions. J Electroanal Chem 385:241–248. https://doi.org/10.1016/0022-0728(94)03788-5
Malik MA, Miecznikowski K, Kulesza PJ (2000) Quartz crystal microbalance monitoring of mass transport during redox processes of cyanometallate modified electrodes: complex charge transport in nickel hexacyanoferrate films. Electrochim Acta 45:3777–3784. https://doi.org/10.1016/S0013-4686(00)00469-2
Chen S-M (2002) Preparation, characterization, and electrocatalytic oxidation properties of iron, cobalt, nickel, and indium hexacyanoferrate. J Electroanal Chem 521:29–52. https://doi.org/10.1016/S0022-0728(02)00677-0
Sun X, Duffort V, Nazar LF (2016) Prussian blue Mg-Li hybrid batteries. Adv Sci 3:1600044. https://doi.org/10.1002/advs.201600044
Ciabocco M, Berrettoni M, Zamponi S, Cox JA, Marini S (2013) Electrochemical behavior of Inhcf in alkali metal electrolytes. J Solid State Electrochem 17:2445–2452. https://doi.org/10.1007/s10008-013-2123-2
Shiga T, Kondo H, Kato Y, Inoue M (2015) Insertion of calcium ion into Prussian blue analogue in nonaqueous solutions and its application to a rechargeable battery with dual carriers. J Phys Chem C 119:27946–27953. https://doi.org/10.1021/acs.jpcc.5b10245
Tojo T, Sugiura Y, Inada R, Sakurai Y (2016) Reversible calcium ion batteries using a dehydrated Prussian blue analogue cathode. Electrochim Acta 207:22–27. https://doi.org/10.1016/j.electacta.2016.04.159
Lipson AL, Han S-D, Kim S, Pan B, Sa N, Liao C, Fister TT, Burrell AK, Vaughey JT, Ingram BJ (2016) Nickel hexacyanoferrate, a versatile intercalation host for divalent ions from nonaqueous electrolytes. J Power Sources 325:646–652. https://doi.org/10.1016/j.jpowsour.2016.06.019
Kuperman N, Padigi P, Goncher G, Evans D, Thiebes J, Solanki R (2017) High performance Prussian blue cathode for nonaqueous Ca-ion intercalation battery. J Power Sources 342:414–418. https://doi.org/10.1016/j.jpowsour.2016.12.074
Gheytani S, Liang Y, Wu F, Jing Y, Dong H, Rao KK, Chi X, Fang F, Yao Y (2017) An aqueous Ca-ion battery. Adv Sci 4:1700465. https://doi.org/10.1002/advs.201700465
Liu S, Pan GL, Li GR, Gao XP (2015) Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries. J Mater Chem A 3:959–962. https://doi.org/10.1039/C4TA04644G
Reed LD, Ortiz SN, Xiong M, Menke EJ (2015) A rechargeable aluminum-ion battery utilizing a copper hexacyanoferrate cathode in an organic electrolyte. Chem Commun 51:14397–14400. https://doi.org/10.1039/c5cc06053b
Wang RY, Shyam B, Stone KH, Weker JN, Pasta M, Lee H-W, Toney MF, Cui Y (2015) Reversible multivalent (monovalent, divalent, trivalent) ion insertion in open framework materials. Adv Energy Mater 5:1401869. https://doi.org/10.1002/aenm.201401869
Padigi P, Goncher G, Evans D, Solanki R (2015) Potassium barium hexacyanoferrate – a potential cathode material for rechargeable calcium ion batteries. J Power Sources 273:460–464. https://doi.org/10.1016/j.jpowsour.2014.09.101
Holland A, Mckerracher RD, Cruden A, Wills RGA (2018) An aluminium battery operating with an aqueous electrolyte. J Appl Electrochem 48:243–250. https://doi.org/10.1007/s10800-018-1154-x
Marzak P, Kosiahn M, Yun J, Bandarenka AS (2019) Intercalation of Mg2+ into electrodeposited Prussian blue analogue thin films from aqueous electrolytes. Electrochim Acta 307:157–163. https://doi.org/10.1016/j.electacta.2019.03.094
Chen L, Bao JL, Dong X, Truhlar DG, Wang Y, Wang C, Xia Y (2017) Aqueous Mg-ion battery based on polyimide anode and Prussian blue cathode. ACS Energy Lett 2:1115–1121. https://doi.org/10.1021/acsenergylett.7b00040
Horanyi G, Inzelt G, Kulesza PJ (1990) Radiotracer study of metal hexacyanometalate films. Sorption of Ca2+ ions into cupric hexacyanoferrate films. Electrochim Acta 35:811–816. https://doi.org/10.1016/0013-4686(90)90073-9
Jayalakshmi M, Gomathi H (2002) Solvation vs hydration of intercalated potassium and sodium ions in Prussian blue films. Bull Electrochem 18:75–80 http://cecri.csircentral.net/id/eprint/1261
Eftekhari A (2004) Potassium secondary cell based on Prussian blue cathode. J Power Sources 126:221–228. https://doi.org/10.1016/j.jpowsour.2003.08.007
Crumbliss AL, Lugg PS, Morosoff N (1984) Alkali metal cation effects in a Prussian blue surface modified electrode. Inorg Chem 23:4701–4708. https://doi.org/10.1021/ic00194a057
Mizuno Y, Okubo M, Hosono E, Kudo T, Zhou H, Oh-ishi K (2013) Suppressed activation energy for interfacial charge transfer of a Prussian blue analog thin film electrode with hydrated ions (Li+, Na+, and Mg2+). J Phys Chem C 117:10877–10882. https://doi.org/10.1021/jp311616s
Scholz F, Dostal A (1996) The formal potentials of solid metal hexacyanometalates. Angew Chem Int Ed 34:2685–2687. https://doi.org/10.1002/anie.199526851
Wagner C, Blank M, Oetken M (2019) Das “Ionenradienparadoxon” – Experimentelle Ermittlung der (hydratisierten) Ionenradien von verschiedenen Kationen durch die Einlagerung in Berliner Blau. CHEMCON 25:57–62. https://doi.org/10.1002/ckon.201800009
Kaplun MM, Smirnov YE, Mikli V, Malev VV (2001) Structure of cobalt hexacyanoferrate films synthesized from a complex electrolyte. Russ J Electrochem 37:914–924. https://doi.org/10.1023/A:1011992109433
Kulesza PJ, Malik MA, Zamponi S, Berrettoni M, Marassi R (1995) Electrolyte-cation-dependent coloring, electrochromism and thermochromism of cobalt(II) hexacyanoferrate(III, II) films. J Electroanal Chem 397:287–292. https://doi.org/10.1016/0022-0728(95)04187-8
Ivanov VD, Alieva AR (2000) Electrochemical behavior of electrode modified with a cobalt hexacyanoferrate film: effect of the supporting electrolyte cation. Russ J Electrochem 36:852–860. https://doi.org/10.1007/BF02757058
Teppen BJ, Miller DM (2006) Hydration energy determines isovalent cation exchange selectivity by clay minerals. Soil Sci Soc Am J 70:31–40. https://doi.org/10.2136/sssaj2004.0212
Rotenberg B, Morel J-P, Marry V, Turq P, Morel-Desrosiers N (2009) On the driving force of cation exchange in clays: insights from combined microcalorimetry experiments and molecular simulation. Geochim Cosmochim Acta 73:4034–4044. https://doi.org/10.1016/j.gca.2009.04.012
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5. – Gibbs free energy of hydration at 298.15 K. J Chem Soc Faraday Trans 87:2995–2999. https://doi.org/10.1039/FT9918702995
Yun J, Pfisterer J, Bandarenka AS (2016) How simple are the models of Na intercalation in aqueous media? Energy Environ Sci 9:955–961. https://doi.org/10.1039/c5ee03197d
Davidson D (1937) The formulation of Prussian blue. J Chem Educ 14:277–281. https://doi.org/10.1021/ed014p277
Chadwick BM, Sharpe AG (1966) Transition metal cyanides and their complexes. Adv Inorg Chem Radiochem 8:83–176. https://doi.org/10.1016/S0065-2792(08)60201-0
Wilde RE, Ghosh SN, Marshall BJ (1970) Prussian blues. Inorg Chem 9:2512–2516. https://doi.org/10.1021/ic50093a027
Kraft A (2008) On the discovery and history of Prussian blue. Bull Hist Chem 33:61–67
Woodward J (1724-1725) IV. Præparatio cærulei prussiaci ex germaniâ missa ad Johannem Woodward, M. D. Prof. Med. Gresh. R. S. S. Phil Trans 33:15–17. https://doi.org/10.1098/rstl.1724.0005
Parry EJ, Coste JH (1896) Commercial Prussian blue. Analyst 21:225–230. https://doi.org/10.1039/AN8962100225
Leeds FH (1897) Notes on Prussian blue. Analyst 22:9–10. https://doi.org/10.1039/AN8972200009
Samain L, Grandjean F, Long GJ, Martinetto P, Bordet P, Sanyova J, Strivay D (2013) Synthesis and fading of eighteenth-century Prussian blue pigments: a combined study by spectroscopic and diffractive techniques using laboratory and synchrotron radiation sources. J Synchrotron Rad 20:460–473. https://doi.org/10.1107/S0909049513004585
Samain L, Grandjean F, Long GJ, Martinetto P, Bordet P, Strivay D (2013) Relationship between the synthesis of Prussian blue pigments, their color, physical properties, and their behavior in paint layers. J Phys Chem C 117:9693–9712. https://doi.org/10.1021/jp3111327
Williamson AW (1845) CLXV. On the blue compounds of cyanogen and iron. Mem Proc Chem Soc 3:125–140. https://doi.org/10.1039/MP8450300125
Williamson AW (1846) Untersuchung einiger Cyanverbindungen des Eisens. Liebigs Ann Chem 57:225–246. https://doi.org/10.1002/jlac.18460570209
Williamson AW (1846) XXVIII. On the blue compounds of cyanogen and iron. Philos Mag 3(29):156–171. https://doi.org/10.1080/14786444608645606
Gay-Lussac JL (1831) Thatsachen zur Geschichte des Berlinerblau’s. Ann Phys (Berlin) 97:490–495. Historical citation is: Poggendorf’s Annalen, 21:490–495. https://doi.org/10.1002/andp.18310970306
Williams HE (1915) The chemistry of cyanogen compounds and their manufacture and estimation. J. & A. Churchill, London
Everitt T (1835) XV. On the reaction which takes place when ferrocyanuret of potassium is distilled with dilute sulphuric acid; with some facts relative to hydrocyanic acid and its preparation of uniform strength. Philos Mag 3 6:97–103. https://doi.org/10.1080/14786443508648542
Hu M, Jiang JS (2011) Facile synthesis of air-stable Prussian white microcubes via a hydrothermal method. Mat Res Bull 46:702–707. https://doi.org/10.1016/j.materresbull.2011.01.017
Skraup ZH (1877) Zur Kenntnis der Eisencyanverbindungen. Liebigs Ann Chem 186:371–388. https://doi.org/10.1002/jlac.18771860212
Reynolds EJ (1887) LXIII. – the composition of Prussian blue and Turnbull's blue. J Chem Soc Trans 51:644–646. https://doi.org/10.1039/CT8875100644
Bonnette AK, Allen JF (1971) Isotopic labeling of Mössbauer studies. Application to the iron cyanides. Inorg Chem 10:1613–1616. https://doi.org/10.1021/ic50102a014
Holtzman H (1945) Alkali resistance of the iron blues. Ind Eng Chem 37:855–861. https://doi.org/10.1021/ie50429a019
Weiser HB, Milligan WO, Bates JB (1942) X-ray diffraction studies on heavy-metal iron-cyanides. J Phys Chem 46:99–111. https://doi.org/10.1021/j150415a013
Armand MB, Whittingham MS, Huggins RA (1972) The iron cyanide bronzes. Mater Res Bull 7:101–107. https://doi.org/10.1016/0025-5408(72)90266-8
Pelouze T-J (1841) Note sur une nouvelle combinaison de cyanogène et de fer. Ann Chim Phys 69:40–43
Wu X, Shao M, Wu C, Qian J, Cao Y, Ai X, Yang H (2016) Low defect FeFe(CN)6 framework as stable host material for high performance Li-ion batteries. ACS Appl Mater Interfaces 8:23706–23712. https://doi.org/10.1021/acsami.6b06880
Yang D, Xu J, Liao X-Z, Wang H, He Y-S, Ma Z-F (2015) Prussian blue without coordinated water as a superior cathode for sodium-ion batteries. Chem Commun 51:8181–8184. https://doi.org/10.1039/C5CC01180A
Li W-J, Chou S-L, Wang J-Z, Kang Y-M, Wang J-L, Liu Y, Gu Q-F, Liu H-K, Dou S-X (2015) Facile method to synthesize Na-enriched Na1+xFeFe(CN)6 frameworks as cathode with superior electrochemical performance for sodium-ion batteries. Chem Mater 27:1997–2003. https://doi.org/10.1021/cm504091z
Wu X, Sun M, Guo S, Qian J, Liu Y, Cao Y, Ai X, Yang H (2015) Vacancy-free Prussian blue nanocrystals with high capacity and superior cyclability for aqueous sodium-ion batteries. Chemnanomat 1:188–193. https://doi.org/10.1002/cnma.201500021
Wu X, Wu C, Wei C, Hu L, Qian J, Cao Y, Ai X, Wang J, Yang H (2016) Highly crystallized Na2CoFe(CN)6 with suppressed lattice defects as superior cathode material for sodium-ion batteries. ACS Appl Mater Interfaces 8:5393–5399. https://doi.org/10.1021/acsami.5b12620
Shi W, Nie P, Shang X, Yang J, Xie Z, Xu R, Liu J (2019) Berlin green-based battery deionization-highly selective potassium recovery in seawater. Electrochim Acta 310:104–112. https://doi.org/10.1016/j.electacta.2019.04.122
Shadike Z, Shi D-R, Wang T, Cao M-H, Yang S-F, Chen J, Fu Z-W (2017) Long life and high-rate Berlin green FeFe(CN)6 cathode material for a non-aqueous potassium-ion battery. J Mater Chem A 5:6393–6398. https://doi.org/10.1039/c7ta00484b
De Wet JF, Rolle R (1965) On the existence and autoreduction of iron(III)-hexacyanoferrate(III), Z. Anorg Allgem Chem 336:96–103. https://doi.org/10.1002/zaac.19653360114
Yang J, Wang H, Lu L, Shi W, Zhang H (2006) Large-scale synthesis of Berlin green Fe[Fe(CN)6] microcubic crystals. Cryst Growth Des 6:2438–2440. https://doi.org/10.1021/cg060469r
Padigi P, Thiebes J, Swan M, Goncher G, Evans D, Solanki R (2015) Prussian green: a high rate capacity cathode for potassium ion batteries. Electrochim Acta 166:32–39. https://doi.org/10.1016/j.electacta.2015.03.084
Dong H, Li Y, Liang Y, Li G, Sun C-J, Ren Y, Lu Y, Yao Y (2016) A magnesium–sodium hybrid battery with high operating voltage. Chem Commun 52:8263–8266. https://doi.org/10.1039/C6CC03081E
Reihlen H, v. Kummer U (1929) Über Komplexe Metallcyanide. II, Liebigs Ann Chem 469:30–44. https://doi.org/10.1002/jlac.19294690105
Messner J (1895) Zur Kenntnis der Ferrocyanide. Z Anorg Allgem Chem 9:126–143. https://doi.org/10.1002/zaac.18950090112
Williams HE (1913) Some green iron cyanogen compounds. Proc Chem Soc London 29:54–55. https://doi.org/10.1039/PL9132900049
Ochmańska J, Kupis D, Galus Z (1994) Redox behaviour and charge transport in solid Berlin green. Microscopic and macroscopic charge diffusion. J Electroanal Chem 371:197–204. https://doi.org/10.1016/0022-0728(93)03230-M
Pajerowski DM, Watanabe T, Yamamoto T, Einaga Y (2011) Electronic conductivity in Berlin green and Prussian blue. Phys Rev B 83:153202. https://doi.org/10.1103/PhysRevB.83.153202
Alich MA, Haworth DT, Johnson MF (1967) Spectrophotometric studies of hexacyanoferrate(III) ion and its reaction with iron(III) in water and ethanol. J Inorg Nucl Chem 29:1637–1642. https://doi.org/10.1016/0022-1902(67)80207-0
Walker RG, Watkins KO (1968) A study of the kinetics of complex formation between hexacyanoferrate(III) ions and iron(III) to form FeFe(CN)6 (Prussian brown). Inorg Chem 7:885–888. https://doi.org/10.1021/ic50063a009
Emrich RJ, Traynor L, Gambogi W, Buhks E (1987) Surface analysis of electrochromic displays of iron hexacyanoferrate films by x-ray photoelectron spectroscopy. J Vac Sci Technol A 5:1307–1310. https://doi.org/10.1116/1.574797
Rosseinsky DR, Glidle A (2003) A EDX, spectroscopy, and composition studies of electrochromic iron(III) hexacyanoferrate(II) deposition. J Electrochem Soc 150:C641–C645. https://doi.org/10.1149/1.1599848
Isfahani VB, Memarian N, Dizaji HR, Arab A, Silva MM (2019) The physical and electrochromic properties of Prussian blue thin films electrodeposited on ITO electrodes. Electrochim Acta 304:282–291. https://doi.org/10.1016/j.electacta.2019.02.120
Chidsey CE, Feldman BJ, Lundgren C, Murray RW (1986) Micrometer-spaced platinum interdigitated array electrode: fabrication, theory, and initial use. Anal Chem 58:601–607. https://doi.org/10.1021/ac00294a026
McCargar JW, Neff VD (1988) Thermodynamics of mixed-valence intercalation reactions: the electrochemical reduction of Prussian blue. J Phys Chem 92:3598–3604. https://doi.org/10.1021/j100323a055
García-Jareño JJ, Navarro-Laboulais J, Vicente F (1997) A numerical approach to the voltammograms of the reduction of Prussian blue films on ITO electrodes. Electrochim Acta 42:1473–1480. https://doi.org/10.1016/S0013-4686(96)00302-7
Agrisuelas J, Bueno PR, Ferreira FF, Gabrielli C, García-Jareño JJ, Gimenez-Romero D, Perrot H, Vicente F (2009) Electronic perspective on the electrochemistry of Prussian blue films. J Electrochem Soc 156:P74–P80. https://doi.org/10.1149/1.3080711
Feldman BJ, Murray RW (1987) Electron diffusion in wet and dry Prussian blue films on interdigitated array electrodes. Inorg Chem 26:1702–1708. https://doi.org/10.1021/ic00258a014
Reguera E, Fernández-Bertrán J, Dago A, Díaz C (1992) Mössbauer spectroscopic study of Prussian blue from different provenances. Hyperfine Interact 73:295–308. https://doi.org/10.1007/BF02418604
Greaves TL, Cashion JD (2016) Site analysis and calculation of the quadrupole splitting of Prussian blue Mössbauer spectra. Hyperfine Interact 237:70–79. https://doi.org/10.1007/s10751-016-1216-6
Matsuda T, Kim J, Moritomo Y (2011) Network dimensionalities and thermal expansion properties of metal nitroprussides. RSC Adv 1:1716–1720. https://doi.org/10.1039/C1RA00547B
Mullica DF, Tippin DB, Sappenfield EL (1991) Synthesis, spectroscopic studies and X-ray crystal structure analysis of cobalt nitroprusside, Co[Fe(CN)5NO]·5H2O. J Coord Chem 24:83–91. https://doi.org/10.1080/00958979109409738
Gómez A, Rodríguez-Hernández J, Reguera E (2007) Crystal structures of cubic nitroprussides: M[Fe(CN)5NO]·xH2O (M = Fe, Co, Ni). Obtaining structural information from the background. Powder Diffraction 22:27–34. https://doi.org/10.1154/1.2700265
de Sá AC, Maraldi VA, Bonfim KS, Souza TR, Paim LL, Barbosa PFP, Nakamura AP, do Carmo DR (2017) Electrocatalitic detection of hydrazine using chemically modified electrodes with cobalt pentacyanonitrosylferrate adsorbed on the 3–aminopropylsilica surface. Int J Chem 9:12–21. https://doi.org/10.5539/ijc.v9n4p12
Paim LL, Stradiotto NR (2010) Electrooxidation of sulfide by cobalt pentacyanonitrosylferrate film on glassy carbon electrode by cyclic voltammetry. Electrochim Acta 55:4144–4147. https://doi.org/10.1016/j.electacta.2010.02.082
Dastangoo H, Poureshghi F (2015) On the ability of metal-nitroprusside complexes as electrode modifiers: characterization and electrochemical study of palladized aluminum electrode modified with iron pentacyanonitrosylferrate. Electrochim Acta 163:271–279. https://doi.org/10.1016/j.electacta.2015.02.003
Paim LL, Hammer P, Stradiotto NR (2011) Electrochemical behavior of a glassy carbon electrode chemically modified with nickel pentacyanonitrosylferrate in presence of sulfur compounds. Electroanalysis 23:1488–1496. https://doi.org/10.1002/elan.201000723
Ellis D, Eckhoff M, Neff VD (1981) Electrochromism in the mixed-valence hexacyanides. 1. Voltammetric and spectral studies of the oxidation and reduction of thin films of Prussian blue. J Phys Chem 85:1225–1231. https://doi.org/10.1021/j150609a026
Rosseinsky DR, Lim H, Jiang H, Chai JW (2003) Optical charge-transfer in iron(III) hexacyanoferrate(II): electro-intercalated cations induce lattice-energy-dependent ground-state energies. Inorg Chem 42:6015–6023. https://doi.org/10.1021/ic020575s
Rosseinsky DR, Glasser L, Jenkins HDB (2004) Thermodynamic clarification of the curious ferric/potassium ion exchange accompanying the electrochromic redox reactions of Prussian blue, iron(III) hexacyanoferrate(II). J Am Chem Soc 126:10472–10477. https://doi.org/10.1021/ja040055r
Agrisuelas J, García-Jareño JJ, Gimenez-Romero D, Vicente F (2009) Insights on the mechanism of insoluble-to-soluble Prussian blue transformation. J Electrochem Soc 156:P149–P156. https://doi.org/10.1149/1.3177258
Malev V, Kurdakova V, Kondratiev V, Zigel V (2004) Indium hexacyanoferrate films, voltammetric and impedance characterization. Solid State Ionics 169:95–104. https://doi.org/10.1016/j.ssi.2003.11.028
Li F, Ma D, Qian J, Yang B, Xu Z, Li D, Wu Z, Wang J (2019) One-step hydrothermal growth and electrochromic properties of highly stable Prussian green film and device. Sol Energy Mater Sol Cells 192:103–108. https://doi.org/10.1016/j.solmat.2018.12.024
Chen R, Huang Y, Xie M, Wang Z, Ye Y, Li L, Wu F (2016) Chemical inhibition method to synthesize highly crystalline Prussian blue analogs for sodium-ion battery cathodes. ACS Appl Mater Interfaces 8:31669–31676. https://doi.org/10.1021/acsami.6b10884
Li C, Zang R, Li P, Man Z, Wang S, Li X, Wu Y, Liu S, Wang G (2018) High crystalline Prussian white nanocubes as a promising cathode for sodium-ion batteries. Chem Asian J 13:342–349. https://doi.org/10.1002/asia.201701715
Brown DB, Shriver DF (1969) Structures and solid-state reactions of Prussian blue analogs containing chromium, manganese, iron, and cobalt. Inorg Chem 8:37–42. https://doi.org/10.1021/ic50071a009
Zentkova M, Mihalik M (2019) The effect of pressure on magnetic properties of Prussian blue analogues. Crystals 9:112. https://doi.org/10.3390/cryst9020112
Mullaliu A, Sougrati M-T, Louvain N, Aquilanti G, Doublet M-L, Stievano L, Giorgetti M (2017) The electrochemical activity of the nitrosyl ligand in copper nitroprusside: a new possible redox mechanism for lithium battery electrode materials? Electrochim Acta 257:364–371. https://doi.org/10.1016/j.electacta.2017.10.107
Acknowledgments
The present author is grateful for all organizations that help to access old scientific works free of charge: the Royal Society, Gallica Bibliotheque Numerique, Google (Google Books), HathiTrust Digital Library, and all others.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivanov, V.D. Four decades of electrochemical investigation of Prussian blue. Ionics 26, 531–547 (2020). https://doi.org/10.1007/s11581-019-03292-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03292-y