Abstract
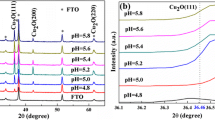
Cu x Co3 − x O4 thin films (with x = 0, 0.1, 0.3, and 0.5) were successfully deposited on glass and indium-doped tin oxide substrates by the sol-gel method. Despite the observed enhancement in crystallinity, a decrease in ion capacity was observed with the increasing of annealing temperature, which was attributed to the suppression of the diffusion of the electrolyte ions into the films. It was also deduced that Cu doping resulted in a significant increase in capacity with a maximum value of 2.92E-02 C/cm2 which was obtained for x = 0.3. The observed decrease in capacity after raising the x value from 0.3 to 0.5 was due to the formation of mixed spinel structure. The results also showed that the variation of optical absorption coefficient, optical band gap energy, electrical resistance, and doping density with x was consistent with the general characteristics of the formed mixed spinel structure when it exceeded x = 0.3.

ᅟ











Similar content being viewed by others
References
Pendashteh A, Moosavifard SE, Rahmanifar MS, Wang Y, El-Kady MF, Kaner RB, Mousavi MF (2015) Highly ordered mesoporous CuCo2O4 nanowires, a promising solution for high-performance supercapacitors. Chem Mater 27:3919–3926
Vigneshwaran P, Kandiban M, Senthil Kumar N, Venkatachalam V, Jayavel R, Vetha Potheher I (2016) A study on the synthesis and characterization of CoMn2O4 electrode material for supercapacitor applications. J Mater Sci : Mater Electron 27:4653–4658
Yu L, Guan B, Xiao W, Lou XW (2015) Formation of yolk-shelled Ni–co mixed oxide nanoprisms with enhanced electrochemical performance for hybrid supercapacitors and lithium ion batteries. Adv Energ Mater 5:1500981-n/a
Wu HB, Pang H, Lou XW (2013) Facile synthesis of mesoporous Ni0.3Co2.7O4 hierarchical structures for high-performance supercapacitors. Energy Environ Sci 6:3619–3626
Brousse T, Bélanger D, Long JW (2015) To be or not to be pseudocapacitive? J Electrochem Soc 162:A5185–A5189
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 343:1210–1211
Yu Z, Tetard L, Zhai L, Thomas J (2015) Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energ Environment Sci 8:702–730
Wang B, Zhu T, Wu HB, Xu R, Chen JS, Lou XW (2012) Porous Co3O4 nanowires derived from long CO(CO3)0.5(OH).0.11H2O nanowires with improved supercapacitive properties. Nano 4:2145–2149
Zhang Y, Ma M, Yang J, Sun C, Su H, Huang W, Dong X (2014) Shape-controlled synthesis of NiCo2S4 and their charge storage characteristics in supercapacitors. Nano 6:9824–9830
Jadhav HS, Pawar SM, Jadhav AH, Thorat GM, Seo JG (2016) Hierarchical mesoporous 3D flower-like CuCo2O4/NF for high-performance electrochemical energy storage. Sci Rep 6:31120
Wang L, Liu X, Wang X, Yang X, Lu L (2010) Preparation and electrochemical properties of mesoporous Co3O4 crater-like microspheres as supercapacitor electrode materials. Curr Appl Phys 10:1422–1426
Reddy MV, Rajesh M, Adams S, Chowdari BVR (2016) Effect of initial reactants and reaction temperature on molten salt synthesis of CuCo2O4 and its sustainable energy storage properties. ACS Sustain Chem Eng 4:3076–3086
Ambare RC, Bharadwaj SR, Lokhande BJ (2014) Electrochemical characterization of Mn: Co3O4 thin films prepared by spray pyrolysis via aqueous route. Curr Appl Phys 14:1582–1590
Liu S, Zhang S, Xing Y, Wang S, Lin R, Wei X, He L (2014) Facile synthesis of hierarchical mesoporous CuxCo3-xO4 nanosheets array on conductive substrates with high-rate performance for Li-ion batteries. Electrochim Acta 150:75–82
Cheng J, Yan H, Lu Y, Qiu K, Hou X, Xu J, Han L, Liu X, Kim J-K, Luo Y (2015) Mesoporous CuCo2O4 nanograsses as multi-functional electrodes for supercapacitors and electro-catalysts. J Mater Chem A 3:9769–9776
Alizadeh-Gheshlaghi E, Shaabani B, Khodayari A, Azizian-Kalandaragh Y, Rahimi R (2012) Investigation of the catalytic activity of nano-sized CuO, Co3O4 and CuCo2O4 powders on thermal decomposition of ammonium perchlorate. Powder Technol 217:330–339
La Rosa-Toro A, Berenguer R, Quijada C, Montilla F, Morallón E, Vázquez JL (2006) Preparation and characterization of copper-doped cobalt oxide electrodes. J Phys Chem B 110:24021–24029
De Koninck M, Poirier S-C, Marsan B (2006) CuxCo3−xO4 used as bifunctional electrocatalyst physicochemical properties and electrochemical characterization for the oxygen evolution reaction. J Electrochem Soc 153:A2103–A2110
Marsan B, Fradette N, Beaudoin G (1992) Physicochemical and electrochemical properties of CuCo2O4 electrodes prepared by thermal decomposition for oxygen evolution. J Electrochem Sci 139:1889–1896
Chi B, Lin H, Li J (2008) Cations distribution of CuxCo3−xO4 and its electrocatalytic activities for oxygen evolution reaction. Int J Hydrogen Energ 33:4763–4768
Zhang K, Zeng W, Zhang G, Hou S, Wang F, Wang T, Duan H (2015) Hierarchical CuCo2O4 nanowire@NiCo2O4 nanosheet core/shell arrays for high-performance supercapacitors. RSC Adv 5:69636–69641
Chen H, Chen X, Zeng Y, Chen S, Wang J (2015) Grass-like CuCo2O4 nanowire arrays supported on nickel foam with high capacitances and desirable cycling performance. RSC Adv 5:70494–70497
Xia X-h, Tu J-p, Zhang Y-q, Mai Y-j, Wang X-l, Gu C-d, Zhao X-b (2012) Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv 2:1835–1841
Gu D, Li W, Wang F, Bongard H, Spliethoff B, Schmidt W, Weidenthaler C, Xia Y, Zhao D, Schüth F (2015) Controllable synthesis of mesoporous peapod-like Co3O4@carbon nanotube arrays for high-performance lithium-ion batteries. Angew Chem Int Edit 54:7060–7064
Liu W, Jiang D, Xia J, Qian J, Wang K, Li H (2014) Preparation of hierarchical mesoporous Co3O4 bundle using [Bmim]TA as a multi-role starting material and its supercapacitor application. Monatsh Chem 145:19–22
Deori K, Ujjain SK, Sharma RK, Deka S (2013) Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl Mater Interface 5:10665–10672
Gao Y, Chen S, Cao D, Wang G, Yin J (2010) Electrochemical capacitance of Co3O4 nanowire arrays supported on nickel foam. J Power Sources 195:1757–1760
Bahlawane N, Fischer Rivera E, Kohse-Höinghaus K, Brechling A, Kleineberg U (2004) Characterization and tests of planar Co3O4 model catalysts prepared by chemical vapor deposition. Appl Catal B-Environ 53:245–255
Abu-Zied BM, Soliman SA, Abdellah SE (2015) Enhanced direct N2O decomposition over CuxCo1−xCo2O4 (0.0 ≤ x ≤ 1.0) spinel-oxide catalysts. J Ind Eng Chem 21:814–821
Guan H, Shao C, Wen S, Chen B, Gong J, Yang X (2003) A novel method for preparing Co3O4 nanofibers by using electrospun PVA/cobalt acetate composite fibers as precursor. Mater Chem Phys 82:1002–1006
Anandha Babu G, Ravi G, Hayakawa Y (2014) Microwave synthesis and effect of CTAB on ferromagnetic properties of NiO, Co3O4 and NiCo2O4 nanostructures. Appl Phys A Mater Sci Process 119:219–232
Wang R, Xu C, Sun J, Liu Y, Gao L, Lin C (2013) Free-standing and binder-free lithium-ion electrodes based on robust layered assembly of graphene and Co3O4 nanosheets. Nano 5:6960–6967
Liotta LF, Wu H, Pantaleo G, Venezia AM (2013) Co3O4 nanocrystals and Co3O4-MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: a review. Catal Sci Tec 3:3085–3102
Ai L, Jiang J (2010) Facile synthesis and characterization of polypyrrole/Co3O4 nanocomposites with adjustable intrinsic electroconductivity. J Mater Sci Mater Electron 21:410–415
Naveen AN, Selladurai S (2016) Novel synthesis of highly porous three-dimensional nickel cobaltite for supercapacitor application. Ionics 22:1471–1483
Luisetto I, Pepe F, Bemporad E (2008) Preparation and characterization of nano cobalt oxide. J Nanopart Res 10:59–67
Chandradass J, Balasubramanian M, Kim KH (2010) Size effect on the magnetic property of CoAl2O4 nanopowders prepared by reverse micelle processing. J Alloy Compd 506:395–399
Connell GAN, Lewis A (1973) Comments on the evidence for sharp and gradual optical absorption edges in amorphous germanium. Phys Status Solidi (b) 60:291–298
Ashour A, Afifi HH, Mahmoud SA (1994) Effect of some spray pyrolysis parameters on electrical and optical properties of ZnS films. Thin Solid Films 248:253–256
Balouria V, Samanta S, Singh A, Debnath AK, Mahajan A, Bedi RK, Aswal DK, Gupta SK (2013) Chemiresistive gas sensing properties of nanocrystalline Co3O4 thin films. Sensor Actuat B-Chem 176:38–45
El-Nahass MM (1992) Optical properties of tin diselenide films. J Mater Sci 27:6597–6604
Yamamoto H, Tanaka S, Hirao K (2003) Effects of substrate temperature on nanostructure and band structure of sputtered Co3O4 thin films. J Appl Phys 93:4158–4162
Amri A, Duan X, Yin C-Y, Jiang Z-T, Rahman MM, Pryor T (2013) Solar absorptance of copper–cobalt oxide thin film coatings with nano-size, grain-like morphology: optimization and synchrotron radiation XPS studies. Appl Surf Sci 275:127–135
Ambrosini A, Lambert TN, Bencomo M, Hall A, Vanevery K, Siegel N, Ho C (2011) Improved high temperature solar absorbers for use in concentrating solar power central receiver applications. Wash DC ASME ES2011-54241:587–594
Tavares AC, da Silva Pereira MI, Mendonça MH, Nunes MR, Costa FM, Sá CM (1998) XPS and voltammetric studies on Ni1−xCuxCo2O4 spinel oxide electrodes. J Electroanal Chem 449:91–100
Liu S, Hui KS, Hui KN, Jadhav VV, Xia QX, Yun JM, Cho YR, Mane RS, Kim KH (2016) Facile synthesis of microsphere copper cobalt carbonate hydroxides electrode for asymmetric supercapacitor. Electrochim Acta 188:898–908
Liu S, Hui KS, Hui KN (2016) Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS Appl Mater Interface 8:3258–3267
Liao Q, Li N, Jin S, Yang G, Wang C (2015) All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 9:5310–5317
Biesinger MC, Payne BP, Grosvenor AP, Lau LWM, Gerson AR, Smart RSC (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257:2717–2730
Shanmugavani A, Selvan RK (2016) Improved electrochemical performances of CuCo2O4/CuO nanocomposites for asymmetric supercapacitors. Electrochim Acta 188:852–862
Gautier JL, Trollund E, Ríos E, Nkeng P, Poillerat G (1997) Characterization of thin CuCo2O4 films prepared by chemical spray pyrolysis. Study of their electrochemical stability by ex situ spectroscopic analysis. J Electroanal Chem 428:47–56
Vijayakumar S, Lee S-H, Ryu K-S (2015) Hierarchical CuCo2O4 nanobelts as a supercapacitor electrode with high areal and specific capacitance. Electrochim Acta 182:979–986
Shelke PN, Khollam YB, Hawaldar RR, Gunjal SD, Udawant RR, Sarode MT, Takwale MG, Mohite KC (2013) Synthesis, characterization and optical properties of selective Co3O4 films 1-D interlinked nanowires prepared by spray pyrolysis technique. Fuel 112:542–549
Li Y, Zhao J, Han J, He X (2005) Combustion synthesis and characterization of NiCuZn ferrite powders. Mater Res Bull 40:981–989
Fradette N, Marsan B (1998) Surface studies of CuxCo3−xO4 electrodes for the electrocatalysis of oxygen evolution. J Electrochem Sci 145:2320–2327
Tyczkowski J, Kapica R, Redzynia W, Kozanecki M, Chehimi MM, Sielski J, Kuberski SM (2013) Plasma deposition and characterization of copper-doped cobalt oxide nanocatalysts. Mater Sci 19:270–276
Angelov S, Tyuliev G, Marinova T (1987) XPS study of surface composition of polycrystalline CuxCo3−xO4 (0⩽x<1) obtained by thermal decomposition of nitrate mixtures. Appl Surf Sci 27:381–392
Guan B, Guo D, Hu L, Zhang G, Fu T, Ren W, Li J, Li Q (2014) Facile synthesis of ZnCo2O4 nanowire cluster arrays on Ni foam for high-performance asymmetric supercapacitors. J Mater Chem A 2:16116–16123
Naveen AN, Manimaran P, Selladurai S (2015) Cobalt oxide (Co3O4)/graphene nanosheets (GNS) composite prepared by novel route for supercapacitor application. J Mater Sci Mater Electron 26:8988–9000
Krishnan SG, Reddy MV, Harilal M, Vidyadharan B, Misnon II, Rahim MHA, Ismail J, Jose R (2015) Characterization of MgCo2O4 as an electrode for high performance supercapacitors. Electrochim Acta 161:312–321
Laban WA, Etgar L (2013) Depleted hole conductor-free lead halide iodide heterojunction solar cells. Energy Environ Sci 6:3249–3253
Kirchartz T, Gong W, Hawks SA, Agostinelli T, MacKenzie RCI, Yang Y, Nelson J (2012) Sensitivity of the Mott–Schottky analysis in organic solar cells. J Phys Chem Soc 116:7672–7680
Liu J, Yang H, Tan W, Zhou X, Lin Y (2010) Photovoltaic performance improvement of dye-sensitized solar cells based on tantalum-doped TiO2 thin films. Electrochim Acta 56:396–400
Acknowledgements
The authors would like to acknowledge the University of Guilan Research Council for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Behzad, H., Ghodsi, F.E. & Karaağaç, H. High electrochemical performance of Cu x Co3−x O4 nanostructured electrodes: the effect of spinel inversion and annealing temperature. Ionics 23, 2429–2442 (2017). https://doi.org/10.1007/s11581-017-2081-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2081-2