Abstract
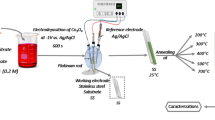
In the present study, copper oxide thin films are deposited using modified successive ionic layer adsorption and reaction (modified SILAR) method on stainless steel (SS) substrates. These deposited CuO films are characterized by different characterization techniques such as X-ray diffraction (XRD). The XRD pattern reveals that CuO films exhibit polycrystalline phase with monoclinic structure which is highly feasible for supercapacitors. The surface morphology was studied by using field emission scanning electron microscopy (FE-SEM) which shows deposited CuO exhibits popcorn-like morphology. The surface wettability study shows prepared CuO is hydrophilic in nature. The electrochemical supercapacitive properties of CuO thin films are evaluated using cyclic voltammetry (CV) in 1 M KOH electrolyte. Which exhibits the maximum specific capacitance of 184 F/g at the scan rate of 50 mV/s also, it shows 83% capacitive retention after 5000 cycles. The values of specific energy and power are gained to be 14.1 and 3 kWk/g, respectively. In addition, impedance measurements of the CuO electrodes show that CuO is the promising material for supercapacitor application.






Similar content being viewed by others
References
Das AK, Maiti S, Khatua BB (2015) High performance electrode material prepared through in-situ polymerization of aniline in the presence of zinc acetate and graphene nanoplatelets for supercapacitor application. J Electroanal Chem 739:10–19. doi:10.1016/j.jelechem.2014.12.018
Nair MTS, Guerrero L, Arenas OL, Nair PK (1999) Chemically deposited copper oxide thin films: structural, optical and electrical characteristics. Appl Surf Sci 150:143–151
Zhang LL, Zhou R, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. J Mater Chem 38:2520–2531. doi:10.1039/c000417k
Krishnamoorthy K, Thangavel S, Chelora Veetil J et al (2016) Graphdiyne nanostructures as a new electrode material for electrochemical supercapacitors. Int J Hydrog Energy 41:1672–1678. doi:10.1016/j.ijhydene.2015.10.118
Pazhamalai P, Krishnamoorthy K, Kim SJ (2016) Hierarchical copper selenide nanoneedles grown on copper foil as a binder free electrode for supercapacitors. Int J Hydrog Energy:1–6. doi:10.1016/j.ijhydene.2016.05.157
Rangom Y, Tang XS, Nazar LF (2015) Carbon nanotube-based supercapacitors with excellent ac line filtering and rate capability via improved interfacial impedance. ACS Nano 9(7):7248–7255. doi:10.1021/acsnano.5b02075
Jiang H, Lee PS, Li C (2013) Three-dimensional carbon based nanostructures for advanced supercapacitors. Energy Environ Sci 6:41–53. doi:10.1039/c2ee23284g
Shi L, Zhang J, Liu H et al (2015) Flower-like Ni(OH)2 hybridized g-C3N4 for high-performance supercapacitor electrode material. Mater Lett 145:150–153. doi:10.1016/j.matlet.2015.01.083
Shinde PA, Lokhande VC, Chodankar NR et al (2016) Enhanced electrochemical performance of monoclinic WO3 thin film with redox additive aqueous electrolyte. J Colloid Interface Sci 483:261–267. doi:10.1016/j.jcis.2016.08.011
Wang K, Dong X, Zhao C et al (2015) Facile synthesis of Cu2O/CuO/RGO nanocomposite and its superior cyclability in supercapacitor. Electrochim Acta 152:433–442. doi:10.1016/j.electacta.2014.11.171
Xu J, Yu K, Wu J, Shang D, Li L, Xu Y, Zhu Z (2009) Synthesis, field emission and humidity sensing characteristics of honeycomb-like CuO. J Phys D Appl Phys. doi:10.1088/0022-3727/42/7/075417
Qin H, Zhang Z, Liu X et al (2010) Room-temperature ferromagnetism in CuO sol-gel powders and films. J Magn Magn Mater 322:1994–1998. doi:10.1016/j.jmmm.2010.01.021
Vila M, Díaz-Guerra C, Piqueras J (2010) Optical and magnetic properties of CuO nanowires grown by thermal oxidation. J Phys D Appl Phys 43. doi:10.1088/0022-3727/43/13/135403
Feng JK, Xia H, Lai MO, Lu L (2011) Electrochemical performance of CuO nanocrystal film fabricated by room temperature sputtering. Mater Res Bull 46:424–427. doi:10.1016/j.materresbull.2010.12.006
Singh I, Bedi RK (2011) Studies and correlation among the structural, electrical and gas response properties of aerosol spray deposited self assembled nanocrystalline CuO. Appl Surf Sci 257:7592–7599. doi:10.1016/j.apsusc.2011.03.133
Mageshwari K, Sathyamoorthy R (2013) Physical properties of nanocrystalline CuO thin films prepared by the SILAR method. Mater Sci Semicond Process 16:337–343. doi:10.1016/j.mssp.2012.09.016
Patil AS, Lohar GM, Fulari VJ (2016) Structural, morphological, optical and photoelectrochemical cell properties of copper oxide using modified SILAR method. J Mater Sci Mater Electron 27:9550–9557. doi:10.1007/s10854-016-5007-2
Dubal DP, Dhawale DS, Salunkhe RR et al (2010) Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J Alloys Compd 492:26–30. doi:10.1016/j.jallcom.2009.11.149
Nwanya AC, Obi D, Ozoemena KI et al (2016) Facile synthesis of nanosheet-like CuO film and its potential application as a high-performance pseudocapacitor electrode. Electrochim Acta 198:220–230. doi:10.1016/j.electacta.2016.03.064
Lamberti A, Fontana M, Bianco S, Tresso E (2016) Flexible solid-state CuxO-based pseudo-supercapacitor by thermal oxidation of copper foils. Int J Hydrog Energy 41:11700–11708. doi:10.1016/j.ijhydene.2015.12.198
Fan Y, Liu P-F, Yang Z-J (2015) CuO nanoparticles supported on carbon microspheres as electrode material for supercapacitors. Ionics (Kiel) 21:185–190. doi:10.1007/s11581-014-1158-4
Bayansal F, Şahin B, Yüksel M, Çetinkara HA (2013) SILAR-based growth of nanostructured CuO thin films from alkaline baths containing saccharin as additive. Mater Lett 98:197–200. doi:10.1016/j.matlet.2013.02.030
Nikam SS, Suryawanshi MP, Bhosale SM et al (2016) Cu2O thin films prepared using modified successive ionic layer adsorption and reaction method and their use in photoelectrochemical solar cells. J Mater Sci Mater Electron 27:1897–1900. doi:10.1007/s10854-015-3970-7
Lohar GM, Jadhav ST, Dhaygude HD et al (2015) Studies of properties of Fe 3þ doped ZnSe nanoparticles and hollow spheres for photoelectrochemical cell application. J Alloys Compd 653:22–31. doi:10.1016/j.jallcom.2015.08.208
Lohar GM, Thombare JV, Shinde SK et al (2014) Structural, photoluminescence and photoelectrochemical properties of electrosynthesized ZnSe spheres. J Mater Sci Mater Electron 25:1597–1604. doi:10.1007/s10854-014-1750-4
Rafea MA, Roushdy N (2009) Determination of the optical band gap for amorphous and nanocrystalline copper oxide thin films prepared by SILAR technique. J Phys D-Applied Phys 42:15413. doi: 10.1088/0022-3727/42/1/015413
Kumbhar VS, Lokhande AC, Gaikwad NS, Lokhande CD (2016) One-step chemical synthesis of samarium telluride thin films and their supercapacitive properties. Chem Phys Lett 645:112–117. doi:10.1016/j.cplett.2015.12.042
Mahadik SA, Pedraza FD, Relekar BP et al (2016) Synthesis and characterization of superhydrophobic–superoleophilic surface. J Sol-Gel Sci Technol 78:475–481. doi:10.1007/s10971-016-3974-7
Lohar GM, Jadhav ST, Takale MV et al (2015) Photoelectrochemical cell studies of Fe2+ doped ZnSe nanorods using the potentiostatic mode of electrodeposition. J Colloid Interface Sci 458:136–146. doi:10.1016/j.jcis.2015.07.046
QT Q, Wang B, Yang LC et al (2008) Study on electrochemical performance of activated carbon in aqueous Li2SO4, Na2SO4 and K2SO4 electrolytes. Electrochem Commun 10:1652–1655. doi:10.1016/j.elecom.2008.08.020
Wen ZB, QT Q, Gao Q et al (2009) An activated carbon with high capacitance from carbonization of a resorcinol-formaldehyde resin. Electrochem Commun 11:715–718. doi:10.1016/j.elecom.2009.01.015
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157:11–27. doi:10.1016/j.jpowsour.2006.02.065
Senthilkumar V, Kim YS, Chandrasekaran S et al (2015) Comparative supercapacitance performance of CuO nanostructures for energy storage device applications. RSC Adv 5:20545–20553. doi:10.1039/C5RA00035A
Gund GS, Dubal DP, Jambure SB et al (2013) Temperature influence on morphological progress of Ni(OH)2 thin films and its subsequent effect on electrochemical supercapacitive properties. J Mater Chem A 1:4793–4803. doi:10.1039/c3ta00024a
Shinde SK, Dubal DP, Ghodake GS et al (2014) Nanoflower-like CuO/Cu(OH)2 hybrid thin films: synthesis and electrochemical supercapacitive properties. J Electroanal Chem 732:80–85. doi:10.1016/j.jelechem.2014.09.004
Pell WG, Conway BE (2001) Analysis of power limitations at porous supercapacitor electrodes under cyclic voltammetry modulation and dc charge. J Power Sources 96:57–67. doi:10.1016/S0378-7753(00)00682-0
Dubal DP, Gund GS, Lokhande CD, Holze R (2013) Decoration of sponge-like Ni ( OH ) 2 nanoparticles onto MWCNTs using an easily manipulated chemical protocol for supercapacitors. ACS Appl Mater Interfaces. doi:10.1021/am3026486
Jagadale AD, Kumbhar VS, Dhawale DS, Lokhande CD (2013) Potentiodynamically deposited nickel oxide (NiO) nanoflakes for pseudocapacitors. J Electroanal Chem 704:90–95. doi:10.1016/j.jelechem.2013.06.020
Jagadale AD, Kumbhar VS, Dhawale DS, Lokhande CD (2013) Performance evaluation of symmetric supercapacitor based on cobalt hydroxide [Co(OH)2] thin film electrodes. Electrochim Acta 98:32–38. doi:10.1016/j.electacta.2013.02.094
Acknowledgements
The authors are thankful to the DST (DST-FIST, DST-PURSE) India for providing instrumental facilities at the Department of Physics, Shivaji University, Kolhapur. One of the authors (A. S. Patil) is thankful to UGC New Delhi for awarding fellowship through the UGC-BSR scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, A.S., Patil, M.D., Lohar, G.M. et al. Supercapacitive properties of CuO thin films using modified SILAR method. Ionics 23, 1259–1266 (2017). https://doi.org/10.1007/s11581-016-1921-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1921-9