Abstract
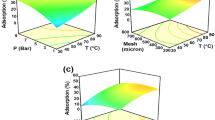
An orthogonal test design was applied to confirm the optimum condition for H2TiO3–lithium adsorbent preparation and Li+ adsorption. Extraction and adsorption mechanism and cycle performance were studied. The verified optimal condition is confirmed as the Li+ concentration, adsorption temperature, molar ratio of Li/Ti, reaction, and pre-calcination temperature are 4.0 g L−1, 60 °C, 2.2, and 650 and 25 °C, respectively. Under the optimal condition, the adsorptive capacity reaches 57.8 mg g−1. Adsorptive capacity of the adsorbent maintains in 5 cycles, typically 25–30 mg g−1.






Similar content being viewed by others
References
Hamzaoui A, Hammi H, M’nif A (2007) Operating conditions for lithium recovery from natural brines. Russ J Inorg Chem 52(12):1859–1863
Van Ginkel SW, Tang Y, Rittmann BE (2011) Impact of precipitation on the treatment of real ion-exchange brine using the H2-based membrane biofilm reactor. Water Sci Technol 63(7):1453
Chitrakar R et al (2014) Lithium recovery from salt lake brine by H 2 TiO 3. Dalton Trans 43(23):8933–8939
Shi X-C et al (2013) Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties. Trans Nonferrous Metals Soc China 23(1):253–259
Tian L, Ma W, Han M (2010) Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide. Chem Eng J 156(1):134–140
Xu C et al (2014) Effect of Cl− on the properties of Li2TiO3 ceramic powders synthesized by in-situ hydrolysis. Ceram Int 40(5):7213–7218
Wu X et al (2008) Optimization of a wet chemistry method for fabrication of Li2TiO3 pebbles. J Nucl Mater 373(1):206–211
Wu X et al (2008) Sol–gel synthesis and sintering of nano-size Li2TiO3 powder. Mater Lett 62(6):837–839
Sinha A, Nair SR, Sinha PK (2010) Single step synthesis of Li2TiO3 powder. J Nucl Mater 399(2):162–166
Ramaraghavulu R, Buddhudu S, Bhaskar Kumar G (2011) Analysis of structural and thermal properties of Li2TiO3 ceramic powders. Ceram Int 37(4):1245–1249
Li Y et al (2012) Synthesis of Li2TiO3 ceramic breeder powders by in-situ hydrolysis and its characterization. Mater Lett 89:25–27
Lee S-J, Park Y-H, Yu M-W (2013) Fabrication of Li2TiO3 pebbles by a freeze drying process. Fusion Eng Des 88(11):3091–3094
Dong D-Q et al (2007) Synthesis of Li 4 Ti 5 O l2 and its exchange kinetics with Li. Acta Phys Chim Sin 23(06):950–954
Deptuła A et al (2009) Preparation of spherical particles of Li2TiO3 (with diameters below 100 μm) by sol–gel process. Fusion Eng Des 84(2):681–684
Laumann A et al (2010) Metastable formation of low temperature cubic Li2TiO3 under hydrothermal conditions—its stability and structural properties. Solid State Ionics 181(33):1525–1529
WU S-C et al (2011) Effect of heat-treatment temperature on the structure and properties of Li_4Ti_5O_ (12) nanorods prepared by the hydrothermal ion exchange method. J Inorg Mater 2:004
Zhang L et al (2014) Effect of crystal phases of titanium dioxide on adsorption performance of H2TiO3-lithium adsorbent. Mater Lett 135:206–209
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56(10):978
Yuan T et al (2010) A mechanism study of synthesis of Li4Ti5O12 from TiO2 anatase. J Alloys Compd 505(1):367–373
Arai H et al (1995) Characterization and cathode performance of Li1 − xNi1 + xO2 prepared with the excess lithium method. Solid State Ionics 80(3–4):261–269
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J Chem Soc, Faraday Trans 87(18):2995–2999
Kataoka K et al (2009) Crystal growth and structure refinement of monoclinic Li2TiO3. Mater Res Bull 44(1):168–172
Vijayakumar M et al (2009) Combined 6, 7Li NMR and molecular dynamics study of Li diffusion in Li2TiO3. J Phys Chem C 113(46):20108–20116
Hosogi Y, Kato H, Kudo A (2008) Visible light response of AgLi 1/3 M 2/3 O 2 (M = Ti and Sn) synthesized from layered Li 2 MO 3 using molten AgNO 3. J Mater Chem 18(6):647–653
Denisova TA et al (2006) Metatitanic acid: synthesis and properties. Russ J Inorg Chem 51(5):691–699
Ariza MJ et al (2006) Probing the local structure and the role of protons in lithium sorption processes of a new lithium-rich manganese oxide. Chem Mater 18(7):1885–1890
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, G., Zhang, L., Zhou, D. et al. The optimal condition for H2TiO3–lithium adsorbent preparation and Li+ adsorption confirmed by an orthogonal test design. Ionics 21, 2219–2226 (2015). https://doi.org/10.1007/s11581-015-1393-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1393-3