Abstract
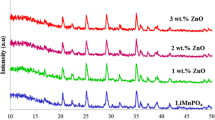
The surface of spinel LiMn2O4 was modified with Fe2O3 (1.0, 2.0, 3.0, 4.0, and 5.0 wt%) by a simple sol-gel method to improve its electrochemical performance at room temperature. Compared with bare LiMn2O4, surface modification improved cycling stability of the material. Among the surface-modified cathode materials, the 3.0- and 4.0-wt% surface-modified cathodes have lesser capacity loss than the others. While the bare LiMn2O4 showed 25.4 % capacity loss in 70 cycles at room temperature, 3.0 and 4.0 wt% of Fe2O3-modified LiMn2O4 only exhibited the capacity loss of 2.6 and 2.3 % in 70 cycles at room temperature, respectively. The structure and phase were identified with X-ray diffractometer along with the lattice constant calculated by a Win-Metric program.










Similar content being viewed by others
References
Linden D, Reddy TB (2002) Handbook of batteries, 3rd edn. McGraw-Hill Inc, New York
Tarascon JM, Armand M (2001) Nature 414:359–367
Whittingham MS (2004) Chem Rev 104:4271–4301
Ronci F, Scrosati B, Rossi A, Perfetti P (2001) J Phys Chem B 105:754–759
Amatucci GG, Pereira N, Zheng T, Plitz I, Tarascon JM (1999) J Power Sources 81–82:39–43
Tsai YW, Santhanam R, Hwang BJ, Hu SK, Sheu HS (2003) J Power Sources 119–121:701–705
Liu W, Farrington GC, Chaput F, Dunn B (1996) J Electrochem Soc 143:879–884
Im D, Manthiram A (2003) J Electrochem Soc 150:A742–A746
Kanamura K, Naito H, Yao T, Takehara Z (1996) J Mater Chem 6:33–36
Oikawa K, Kamiyama T, Izumi F, Chakoumakos BC, Ikuta H, Wakihara M, Li JQ, Matsui Y (1998) Solid State Ion 109:35–41
Song MY, Ahn DS, Park HR (1999) J Power Sources 83:57–60
Sun YK, Jeon YS, Lee HJ (2000) Electrochem Solid-State Lett 3:7–10
Benedek R, Thackeray MM (2006) Electrochem. Solid State Lett 9:A265–267
Aurbach D, Markovsky B, Salitra G, Markevich E, Talyossef Y, Koltypin M, Nazar L, Ellis B, Kovacheva D (2007) J Power Sources 165:491–499
Nakayama N, Nozawa T, Iriyama Y, Abe T, Ogumi Z, Kikuchi K (2007) J Power Sources 174:695–700
Ouyang CY, Shi SQ, Lei MS (2009) J Alloys Compd 474:370–374
Sun YK, Kim DW, Choi YM (1999) J Power Sources 79:231–237
Shi S, Ouyung C, Wang DS, Chen L (2003) Solid State Commun 126:531–534
Dokko K, Hovikoshi S, Itoh T, Nishizawa M, Mohamedi M (2000) J Power Sources 90:109–115
Hosoya M, Ikuta H, Wakihara M (1998) Solid State Ion 111:153–159
Bang HJ, Donepudi VS, Prakash J (2002) Electrochim Acta 48:443–451
Amine K, Tukamoto H, Yasuda H, Fujita Y (1997) J Power Sources 68:604–608
Komaba S, Oikawa K, Myung S-T, Kumagai N, Kamiyama T (2002) Solid State Ion 149:47–52
Amatucci GG, Blyr A, Sigala C, Alfonse P, Tarascon JM (1997) Solid State Ion 104:13–25
Şahan H, Göktepe H, Patat Ş, Ülgen A (2008) Solid State Ion 178:1837–1842
Lee SW, Kim KS, Moon HS, Kim HJ, Cho BW (2004) J Power Sources 126:150–155
Şahan H, Göktepe H, Patat Ş, Ülgen A (2010) Solid State Ion 18:1437–1444
Alcantara R, Jaraba M, Larcla P, Tirado JL (2004) J Electroanal Chem 566:187–192
Tu J, Zhao XB, Xie J, Cao GS, Zhuang DG (2007) J Alloy Compd 432:313–317
Yu L, Qiu X, Xi J, Zhu W, Chen L (2006) Electrochim Acta 51:6406–6411
Eftekhari A (2004) J Power Sources 130:260–265
Cho J, Kim YW, Kim B, Park B (2003) Chem, Int Ed 42:1618–1621
Liu D, He Z, Liu X (2007) Mater Lett 61:4703–4706
Ha HW, Yan NJ, Kim K (2007) Electrochim Acta 52:3236–3241
Sun YC, Wang ZX, Huang XJ (2003) J Electrochem Soc 150:A1294–A1298
Arumugam D, Kalaignan GP (2008) J Electroanal Chem 624:197–204
Lim S, Cho J (2008) Electrochem Commun 10:1478–1481
Lee KS, Myung ST, Bang H, Amine K, Kim DW, Sun YK (2009) J Power Sources 189:494–498
Şahan H, Göktepe H, Patat S, Ülgen A (2011) J Alloys Compd 509:4235–4241
Şahan H, Göktepe H, Patat S (2011) J Mater Sci Technol 27:415–420
Groysman A (2010) Corrosion for everybody. Springer, New York
Zhang Y, Shin HC, Dong J, Liu M (2004) Solid State Ion 171:25–31
Armarego WLF, Perrin DD (2002) Purification of laboratory chemicals, 4th edn. Butterworth Heinemann, Oxford
Cho NW, Chang S, Sung HP (1997) RIST Yongu Nonmun 11:622
Shannon RD (1976) Acta Cryst A32:751–767
Ohzuku T, Ariyoshi K, Takeda S, Sakai Y (2001) Electrochim Acta 46:2327–2336
Kanoh H, Tang W, Ooi K (1998) Electrochem Solid-State Lett 1:17
Julien CM, Massot M (2003) Mater Sci Eng B97:217–230
Sigala C, Guyomard D, Verbaere A, Piffard Y, Tournoux M (1995) Solid State Ion 81:167–170
Arumugam D, Paruthimal G, Kalaignan P (2010) Mater Res Bull 45:1825–1831
Park SB, Shin HC, Lee WG, Cho W, Jang H (2008) J Power Sources 180:597–601
Lee SW, Kim KS, Lee KL, Moon HS, Kim HJ, Cho BW, Cho W, Park JW (2004) J Power Sources 130:233–240
Jang DH, Shin YJ, Oh SM (1996) J Electrochem Soc 143:2204–2211
Xia Y, Zhou Y, Yoshio M (1997) J Electrochem Soc 144:2593–2600
Myung ST, Izumi K, Komaba S, Sun YK, Yashiro H, Kumagai N (2005) Chem Mater 7:3695–3704
Myung ST, Hosoya K, Komaba S, Yashiro H (2006) Electrochim Acta 51:5912–5919
Tarascon JM, Kinnon RC, Coowar F, Bowmer TN (1997) J Electrochem Soc 141:1421–1433
Li C, Zhang HP, Fu LJ, Liu H, Wu YP, Rahm E, Holze R, Wu HQ (2006) Electrochim Acta 51:3872–3883
Acknowledgments
This study was financially supported by the Research Foundation of Erciyes University (FBA-08-439) and International Post Doctoral Research Fellowship Programme (2219) of The Scientific and Technological Research Council of Turkey. The authors would like to thank Mr. İhsan Akşit for the SEM observation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Şahan, H., Dokan, F.K., Ülgen, A. et al. Improvement of cycling stability of LiMn2O4 cathode by Fe2O3 surface modification for Li-ion battery. Ionics 20, 323–333 (2014). https://doi.org/10.1007/s11581-013-0987-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0987-x