Abstract
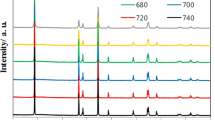
LiNi0.5Mn1.5O4 cathodes were synthesized by three different raw materials at high temperature. The samples were characterized by X-ray diffraction and scanning electron microscopy tests, respectively. The results indicate that the synthesized samples show pure spinel structure, and the samples synthesized by nickel–manganese hydrate and nickel–manganese oxide show regular geometrical shape. The electrochemical performance of sample synthesized by nickel–manganese oxide is best. The first discharge capacity is 141 mAh/g, and the capacity retention is 98.6% after 50 cycles at 0.5 C rate. The discharge capacity at 5 C rate is still 120 mAh/g. Better crystallization, smaller specific surface area, and lower polarization may be responsible for the excellent electrochemical performance of the LiNi0.5Mn1.5O4.






Similar content being viewed by others
References
Kumar G, Schlorb H, Rahner D (2001) Mater Chem Phys 70:117–121
Kawai H, Nagagta M, Kageyama H, Tsukamoto H, West AR (1999) Electrochim Acta 45:315–319
Shigemura H, Sakaebe H, Kageyama H, Kobayashi H, West AR, Kanno R, Morimoto S, Nasu S, Tabuchi M (2001) J Electrochem Chem 148:A730–733
Ein-Eli Y, Urian RC, Wen W, Mukerjee S (2005) Electrochim Acta 50:1931–1935
Fang HS, Wang ZX, Li XH, Guo HJ, Peng WJ (2006) J Power Sources 153:174–176
Fang HS, Wang ZX, Zhang B, Li XH, Li GS (2007) Electrochem Comm 9:1077–1079
Lafonta U, Locati C, Borghols WJ, Łasinska A, Dygas J, Chadwickd AV, Kelder EM (2009) J Power Sources 189:179–182
Xu XX, Yang J, Wang YQ, NuLi YN, Wang JL (2007) J Power Sources 174:1113–1116
Zhang XF, Liu J, Yu HY, Yang GL, Wang JW, Yu ZJ, Xie HM, Wang RS (2010) Electrochim Acta 55:2414–2418
Myung ST, Komaba S, Kumagai N, Yashiro H, Chung HT, Cho TH (2002) Electrochim Acta 47:2543–2547
Wen L, Lu Q, Xu GX (2006) Electrochim Acta 51:4388–4392
Zhang L, Lv XY, Wen YX, Wang F, Su HF (2009) J Alloys Compd 480:802–805
Park SH, Sun YK (2004) Electrochim Acta 50:431–434
Sebastien P, Lucas S, Helene L, Yvan R, Carole B (2008) Electrochim Acta 53:4137–4140
Alcantara R, Jaraba M, Lavela P (2002) Electrochim Acta 47:1829–1832
Ji Y, Wang ZX, Yin ZL, Guo HJ, Peng WJ, Li XH (2007) Chin J Inorg Chem 23:597–601
Chen ZY, Xiao J, Zhu HL, Liu YX (2005) Chin J Inorg Chem 21:1417–1420
Zhang NQ, Yang TY, Lang Y, Sun KN (2011) J Alloys Compd 509:3783–3786
Takahashi K, Saitoh M, Sano M, Fujita M, Kifune K (2004) J Electrochem Soc 151:A173–176
Alcantara R, Jaraba M, Lavela P, Tirada JL (2002) Electrochim Acta 47:1829
Zhang ZR, Liu HS, Gong ZL, Yang Y (2004) J Power Sources 129:101–104
Acknowledgments
The authors gratefully acknowledge the Natural Science Foundation of Jiangsu Province (BK2011530) and the Advanced Foundation of Jiangsu University (10JDG041).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, L. Study on the electrochemical performance of LiNi0.5Mn1.5O4 with different precursor. Ionics 18, 649–653 (2012). https://doi.org/10.1007/s11581-012-0670-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0670-7