Abstract
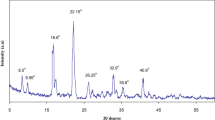
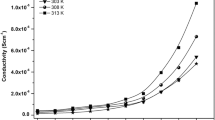
The ZnO filler has been introduced into a solid polymeric electrolyte of polyvinyl chloride (PVC)–ZnO–LiClO4, replacing costly organic filler for conductivity improvement. Ionic conductivity of PVC–ZnO–LiClO4 as a function of ZnO concentration and temperature has been studied. The electrolyte samples were prepared by solution casting technique. The ionic conductivity was measured using impedance spectroscopy technique. It was observed that the conductivity of the electrolyte varies with ZnO concentration and temperature. The temperature dependence on the conductivity of electrolyte was modelled by Arrhenius and Vogel–Tammann–Fulcher equations, respectively. The temperature dependence on the conductivity does not fit in both models. The highest room temperature conductivity of the electrolyte of 3.7 × 10−7 Scm−1 was obtained at 20% by weight of ZnO and that without ZnO filler was found to be 8.8 × 10−10 Scm−1. The conductivity has been improved by 420 times when the ZnO filler was introduced into the PVC–LiClO4 electrolyte system. It was also found that the glass transition temperature of the electrolyte PVC–ZnO–LiClO4 is about the same as PVC–LiClO4. The increase in conductivity of the electrolyte with the ZnO filler was explained in terms of its surface morphology.







Similar content being viewed by others
References
Rahman MYA, Salleh MM, Talib IA, Yahaya M (2002) Solid state ionics: trends in the new millennium. Langkawi, Malaysia, Dec 15–19 2002
Rahman MYA, Salleh MM, Talib IA, Yahaya M (2004) J Power Sources 133:293
Xiong HM, Zhao KK, Zhao X, Wang YW, Chen JS (2003) Solid State Ion 159:89
Fan L, Nan CW, Zhao S (2003) Solid State Ion 164:81
Kim KM, Ko JM, Park NG, Ryu KS, Chang SH (2003) Solid State Ion 161:121
Singh ThJ, Bhat SV (2004) J Power Sources 129:280
Ahmad A, Rahman MYA, Ali MLM, Hashim H, Kalam FA (2007) Ionics 13:67
Janaki Rami Reddy T, Achari VBS, Sharma AK, Narasimha Rao VVR (2007) Ionics 13:55
Park CH, Kim DW, Prakash J, Sun YK (2003) Solid State Ion 159:111
Subramania A, Kalyana Sundaram NT, Vijaya Kumar G (2006) J Power Sources 153:177
Rajendran S, Sivakumar P, Shanker Babu R (2006) J Power Sources 164:815
Rocco AM, Fonseca CP, Loureiro FAM, Pereira RP (2004) Solid State Ion 166:115
Kang Y, Lee J, Lee J, Lee C (2007) J Power Sources 165:92
Itoh T, Hamaguchi Y, Uno T, Kubo M, Aihara Y, Sonai A (2006) Solid State Ion 177:185
Mohamad AA, Arof AK (2006) Ionics 12:57
Xuping Z, Lianyong S, Hua H, Hongli L, Zuhong L (1999) J Mater Sci Lett 18:1745
Acknowledgement
The author is very thankful to the School of Food Technology and Chemical Sciences, Faculty of Science and Technology, UKM for sample preparation and characterisation. This work was funded by UNITEN under the internal grant no. J510010250.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.Y.A., Ahmad, A. & Wahab, S.A. Electrical properties of a solid polymeric electrolyte of PVC–ZnO–LiClO4 . Ionics 15, 221–225 (2009). https://doi.org/10.1007/s11581-008-0262-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-008-0262-8