Abstract
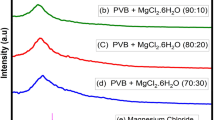
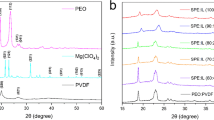
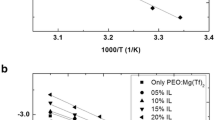
A solid polymer electrolyte (SPE) film consisting of poly(ethylene oxide) (PEO) with magnesium chloride as electrolytic salt and B2O3 as the filler has been prepared by solution casting technique. The polymeric film was flexible and self-standing with proper mechanical strength and studied for application in a solid-state rechargeable magnesium battery. The interactions between the filler and PEO chains are studied by differential scanning calorimeter and Fourier transform infrared techniques. Composition of SPE is optimized, and maximum conductivity is obtained at 2 wt% B2O3. Filler seems to increase the number of free magnesium cations by decoordinating the bond between magnesium cations and ether oxygen of PEO. Cyclic voltammetry results show the reversible capability of magnesium electrode. Solid-state magnesium cell employing magnesium anode, SPE, and manganese oxide was assembled, and its open circuit voltage is found to be 1.9 V.





Similar content being viewed by others
References
Murata K (1995) Electrochim Acta 40:2177
Hashmi SA, Chandra A, Chandra S (1992) In: B.V.R. Chowdari, et al (eds) Solid state ionics: materials and applications. World Scientific, Singapore, p 567
Sreepathi Rao S, Jaipal Reddy M, Laxmi Narsaiah E, Subba Rao UV (1995) Mater Sci Eng B 33:173
Gorecki W, Jeannin M, Belorizky E, Roux C, Armand M (1995) J Phys Condens Matter 7:6823
Selladurai S, Trends in solid state Ionics, Raj Publishing House, Chennai
Sreekanth T, Jaipal Reddy M, Subramanyam S, Subba Rao UV (1999) Mater Sci Eng B 64:107
Narin K, Forsyhn M (1996) Solid State Ion 86–88:589
Michael MS, Jacob MME, Prabaharan SSR (1997) Solid State Ion 98:167
Alexandre M, Dubois P (2000) Material Science and Engineering 28:1–63
Yamamoto M et al (2003) J Mater Chem 13:280–285
Hashmi SA, Kumar A, Maurya KK, Chandra S (1990) J Phys D 23:784
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nature 394:456
Croce F, Curini R, Martinelli A, Persi L, Ronci F, Scrosati B (1999) J Phys Chem B 103:10632
Ji et al (2003) J Power Sources 117:124–130
Doeff M, Georen P, Qiao J, Kerr J, De Jonghe LC (1999) J Electrochim Soc 146:2024
Dupon R, Papke BL, Ratner MA, Whitmore DH, Shriver DF (1982) J Am Chem Soc 104:6247
Kumar B, Stanley J, Rodrigues RJ (2002) Spry. Electrochim Acta 47:1275–1281
Hench LL, West JK (1990) Principles of electronic of ceramics. Wiley, New York, USA, pp 139–198
Wunderlich (ed) (1980) Macromolecular physics, vol 3. Academic, New York, p 67
Girish Kumar G, Munichandraiah N (2000) J Electroanal Chem 495:42–50
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundar, M., Selladurai, S. Effect of fillers on magnesium–poly(ethylene oxide) solid polymer electrolyte. Ionics 12, 281–286 (2006). https://doi.org/10.1007/s11581-006-0048-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-006-0048-9